Metabolite Profiling and Antioxidant Activity of 10 New Early- to Mid-Season Apple Cultivars and 14 Traditional Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apple Samples
2.3. Total Soluble Solid Content
2.4. Free Sugar and Organic Acid Content of Apple Juice
2.5. Extraction Procedure for Analyzing Antioxidant Activities and Phenolic Composition of Apple Samples
2.6. Antioxidant Activities
2.7. Determination of Phenolic Compounds Using UHPLC-(ESI)-qTOF and HPLC-PDA Detector
2.8. Statistical Analysis
3. Results and Discussion
3.1. Free Sugar and Organic Acid Content of Apple Juice
3.2. Antioxidant Activity
3.3. Identification of Phenolic Compounds in the Apple Peel and Pulp by UHPLC-(ESI)-qTOF
3.4. Relative Quantification of Phenolic Compounds in the Apple Peel and Pulp by UHPLC-(ESI)-qTOF
3.5. Quantification of Phenolic Compounds in the Apple Peel and Pulp by HPLC-PDA
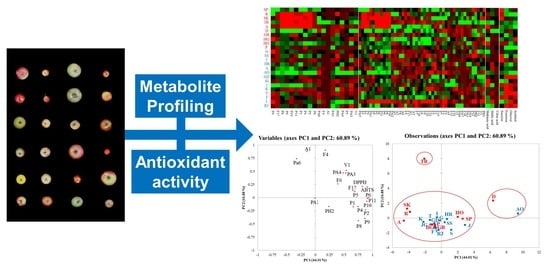
3.6. Principal Component Analysis (PCA) Heatmap Result
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Korean Statistical Information Service. Available online: http://kosis.kr/index/index.do (accessed on 13 August 2019).
- Xiao, G.; Zhang, Q.; Wang, R.; Yao, Y.; Zhao, H.; Bai, H.; Xiong, Y. Effects of temperature increase on pea production in a semiarid region of China. Air Soil Water Res. 2020, 2. [Google Scholar] [CrossRef]
- Grab, S.; Craparo, A. Advance of apple and pear tree full bloom dates in response to climate change in the southwestern Cape, South Africa: 1973–2009. Agric. For. Meteorol. 2011, 151, 406–413. [Google Scholar] [CrossRef]
- Warrington, I.; Fulton, T.; Halligan, E.; De Silva, H. Apple fruit growth and maturity are affected by early season temperatures. J. Am. Soc. Hortic. Sci. 1999, 124, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Skreli, E.; Imami, D. Analyzing consumers’ preferences for apple attributes in Tirana, Albania. Int. Food Agribus. Manag. 2012, 15, 137–155. [Google Scholar]
- Sugiura, T.; Ogawa, H.; Fukuda, N.; Moriguchi, T. Changes in the taste and textural attributes of apples in response to climate change. Sci. Rep. 2013, 3, 2418. [Google Scholar] [CrossRef] [Green Version]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Morton, L.W.; Caccetta, R.A.A.; Puddey, I.B.; Croft, K.D. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2000, 27, 152–159. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kim, S.S.; Mitchell, A.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food. Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R.; Yang, R.; Christopher, J.; Zhu, Y.; Zhu, H.H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Napolitano, A.; Cascone, A.; Graziani, G.; Ferracane, R.; Scalfi, L.; Di Vaio, C.; Ritieni, A.; Fogliano, V. Influence of variety and storage on the polyphenol composition of apple flesh. J. Agric. Food Chem. 2004, 52, 6526–6531. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. Int. J. Food Sci. Technol. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Łysiak, G.; Michalska-Ciechanowska, A.; Wojdyło, A. Postharvest changes in phenolic compounds and antioxidant capacity of apples cv. Jonagold growing in different locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, M.C.; Ku, K.H. Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. The influence of exposure to light on the phenolic content of ‘Fuji’ apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.C.; Copetti, C.; Di Pietro, P.F.; Nunes, E.D.; Fett, R. Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Sari, M.; Chung, Y.; Agatha, F.; Kim, H.K. Evaluation of antioxidant and antimicrobial activity of phenolic lipids produced by the transesterification of 4-hydroxyphenylacetic acid and triglycerides. Appl. Biol. Chem. 2019, 62, 5. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberic, R.; Trobec, M.; Toplak, H.; Stampar, F.; Keppel, H.; Grill, D. Sugar-, acid- and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Bowen, J.H.; Watkins, C.B. Fruit maturity, carbohydrate and mineral content relationships with watercore in ‘Fuji’ apples. Postharvest Biol. Technol. 1997, 11, 31–38. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Echeverria, G.; Puy, J. Segregation of peach and nectarine (Prunus persica (L.) Batsch) cultivars according to their organoleptic characteristics. Postharvest Biol. Technol. 2006, 39, 10–18. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Lachowicz, S.; Glawdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Otagaki, S.; Saelai, P.; Kondo, S.; Shiratake, K.; Matsumoto, S. Varietal differences in phenolic compounds metabolism of type 2 red-fleshed apples. Sci. Hortic. 2017, 219, 1–9. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macia, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with traditional and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Mari, A.; Tedesco, I.; Nappo, A.; Russo, G.L.; Malorni, A.; Carbone, V. Phenolic compound characterisation and antiproliferative activity of “Annurca” apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Huber, G.M.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, C693–C700. [Google Scholar] [CrossRef]
- Alarcon-Flores, M.I.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich, A.G. Evaluation of the presence of phenolic compounds in different varieties of apple by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Food Anal. Meth. 2015, 8, 696–709. [Google Scholar] [CrossRef]
- Sawa, T.; Nakao, M.; Akaike, T.; Ono, K.; Maeda, H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. J. Agric. Food Chem. 1999, 47, 397–402. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stockl, A.; Bohm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Kim, Y.J.; Kim, D.-O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.J.; Sun, H.D.; Xu, Y.; Wang, T.T.; Zhao, R.X. Comparison of antioxidant activities and high-performance liquid chromatography analysis of polyphenol from different apple varieties. Int. J. Food Prop. 2016, 19, 2396–2407. [Google Scholar] [CrossRef] [Green Version]
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; DeEll, J. Phenolic composition and antioxidant activity of selected apple genotypes. J. Food Agric. Environ. 2007, 5, 61–66. [Google Scholar]
- Escarpa, A.; Gonzalez, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Zheng, H.-Z.; Kim, Y.-I.; Chung, S.-K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. Technol. 2009, 10, 512–516. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Taghizadeh, S.F.; Davarynejad, G.; Asili, J.; Nemati, S.H.; Karimi, G. Assessment of phenolic profile and antioxidant power of five pistachio (Pistacia vera) cultivars collected from four geographical regions of Iran. Avicenna J. Phytomed. 2018, 8, 33–42. [Google Scholar] [PubMed]





| Cultivar | Sample Code | Peel Color | Registration Year | Genetic Information | Harvest Season | New/Traditional | Fruit Weight (g) | Soluble Solid Content (SSC, °Brix) |
|---|---|---|---|---|---|---|---|---|
| Summer Prince | SP | red | 2014 | Tsugaru (IT 253737) × OBIR2T47 (IT 250090) | early to mid | new | 293 | 13.4 ± 0.0 f |
| (early Jul) | ||||||||
| Ruby-S | R | Red | 2014 | Alps Otome (IT 249906) × Sansa (IT 225509) | early to mid | new | 97 | 15.7 ± 0.0 n |
| (late Aug) | ||||||||
| Summer King | SK | Yellow | 2010 | Fuji × Golden Delicious | early to mid | new | 313 | 10.9 ± 0.0 b |
| (late Aug) | ||||||||
| Tinkerbell | TB | Red | 2012 | Hongro (IT225596) | early to mid | new | 120 | 15.9 ± 0.0 o |
| (late Aug) | ||||||||
| Arisoo | A | Red | 2011 | Yoko × Shensu | early to mid | new | 305 | 15.3 ± 0.1 m |
| (early Sept) | ||||||||
| Decobell | D | red | 2013 | Sansa (IT225509) × JayDarling (IT225830) | early to mid | new | 21 | 13.5 ± 0.1 g |
| (early Sept) | ||||||||
| Green Ball | GB | green and yellow | 2008 | Golden Delicious × Fuji | early to mid | new | 318 | 15.1 ± 0.1 l |
| (early Sept) | ||||||||
| Honggeum | HG | red | 2004 | Shensu × Hongro | early to mid | new | 360 | 12.8 ± 0.0 d |
| (early Sept) | ||||||||
| Hwangok | HO | yellow | 2009 | Hongwol × Yataka Fuji | early to mid | new | 242 | 11.9 ± 0.0 c |
| (late Sept) | ||||||||
| Picnic | P | red | 2008 | Fuji × Sansa (IT225509) | early to mid | new | 218 | 16.1 ± 0.1 p |
| (late Sept) | ||||||||
| Gala | G | red and yellow | 1960 | Kidd’s Orange Red × Golden Delicious | early to mid | traditional | 266 | 12.8 ± 0.1 d |
| (late Aug) | ||||||||
| Sansa | SS | red | 1986 | Gala × Akane | early to mid | traditional | 239 | 13.7 ± 0.0 h |
| (late Aug) | ||||||||
| Tsugaru | T | red and green | 1975 | Golden Delicious × Jonathan | early to mid | traditional | 261 | 10.0 ± 0.1 a |
| (late Aug) | ||||||||
| Hongro | HR | red | 1988 | Supr Earliblaze × Supr Golden Delicious | early to mid | traditional | 282 | 13.2 ± 0.0 e |
| (early Sept) | ||||||||
| Sekaiichi | S | red | 1974 | Golden Delicious × Red Delicious | early to mid | traditional | 430 | 12.8 ± 0.0 d |
| (late Sept) | ||||||||
| Alps Otome | AO | red | 1968 | − | early to mid | traditional | 38 | 15.6 ± 0.0 n |
| (early Oct) | ||||||||
| Golden Delicious | GD | yellow | 1914 | − | early to mid | traditional | 254 | 13.9 ± 0.1 i |
| (early Oct) | ||||||||
| Jonagold | JG | red and green | 1968 | Jonathan × Golden Delicious | early to mid | traditional | 261 | 16.3 ± 0.0 q |
| (early Oct) | ||||||||
| Jonathan | J | red | 1826 | Espopus Spitzenberg seedling (found in USA) | early to mid | traditional | 185 | 13.5 ± 0.1 g |
| (early Oct) | ||||||||
| Kamhong | K | red | 1992 | Supr Earliblaze × Supr Golden Delicious | early to mid | traditional | 356 | 14.3 ± 0.0 k |
| (early Oct) | ||||||||
| Yoko | Y | red | 1981 | Golden Delicious × unknown pollen parent | early to mid | traditional | 243 | 13.2 ± 0.1 e |
| (possibly Jonathan) | (early Oct) | |||||||
| Fuji | F | red | 1962 | Rall’s Janet × Red Delicious | late (late Oct) | traditional | 242 | 15.4 ± 0.0 m |
| Indo | I | green | 1868 | − | late (early Nov) | traditional | 247 | 15.2 ± 0.1 l |
| Ralls Janet | RJ | red | 1800 | − | late (early Nov) | traditional | 182 | 14.1 ± 0.0 j |
| Peel | Pulp | |||
|---|---|---|---|---|
| Variety | DPPH | ABTS | DPPH | ABTS |
| Summer Prince | 0.71 ± 0.01 fghi | 3.87 ± 0.01 f | 0.48 ± 0.02 J | 1.95 ± 0.05 IJ |
| Ruby-S | 0.41 ± 0.04 b | 2.50 ± 0.05 a | 0.18 ± 0.01 C | 0.54 ± 0.07 B |
| Summer King | 0.28 ± 0.04 a | 2.49 ± 0.04 a | 0.11 ± 0.01 B | 0.41 ± 0.05 B |
| Tinkerbell | 0.92 ± 0.03 k | 4.98 ± 0.07 i | 0.56 ± 0.03 LM | 2.10 ± 0.05 K |
| Arisoo | 0.54 ± 0.03 d | 3.51 ± 0.13 de | 0.03 ± 0.00 A | 0.23 ± 0.06 A |
| Decobell | 1.25 ± 0.03 m | 7.70 ± 0.07 j | 0.87 ± 0.04 O | 4.20 ± 0.07 N |
| Green Ball | 0.56 ± 0.01 d | 3.26 ± 0.06 bc | 0.28 ± 0.04 D | 1.17 ± 0.04 D |
| Honggeum | 0.77 ± 0.00 j | 4.30 ± 0.03 g | 0.35 ± 0.02 EF | 1.41 ± 0.07 E |
| Hwangok | 0.76 ± 0.02 ij | 4.32 ± 0.10 g | 0.45 ± 0.02 IJ | 1.81 ± 0.03 FGH |
| Picnic | 0.71 ± 0.02 fghi | 3.56 ± 0.18 e | 0.53 ± 0.03 KL | 1.83 ± 0.01 FGHI |
| Gala | 0.73 ± 0.04 ghij | 4.24 ± 0.14 g | 0.42 ± 0.03 GHI | 1.75 ± 0.07 FG |
| Sansa | 0.98 ± 0.03 l | 4.53 ± 0.13 h | 0.56 ± 0.02 LM | 2.15 ± 0.05 KL |
| Tsugaru | 0.79 ± 0.03 j | 3.26 ± 0.11 bc | 0.38 ± 0.04 EFG | 1.09 ± 0.18 CD |
| Hongro | 1.23 ± 0.04 m | 3.93 ± 0.18 f | 0.63 ± 0.04 N | 1.85 ± 0.01 GHI |
| Sekaiichi | 0.92 ± 0.03 k | 4.57 ± 0.08 h | 0.59 ± 0.04 MN | 2.03 ± 0.07 JK |
| Alps Otome | 0.92 ± 0.03 k | 5.04 ± 0.08 i | 0.83 ± 0.06 O | 3.96 ± 0.03 M |
| Golden Delicious | 0.51 ± 0.02 cd | 3.34 ± 0.04 cd | 0.32 ± 0.02 DE | 1.48 ± 0.10 E |
| Jonagold | 0.65 ± 0.05 e | 3.98 ± 0.05 f | 0.44 ± 0.04 HIJ | 1.76 ± 0.05 FG |
| Jonathan | 0.70 ± 0.04 efg | 4.29 ± 0.20 g | 0.39 ± 0.0.4 FGH | 2.25 ± 0.09 L |
| Kamhong | 0.75 ± 0.02 hij | 3.92 ± 0.09 f | 0.35 ± 0.05 EF | 1.02 ± 0.06 C |
| Yoko | 0.66 ± 0.04 ef | 3.51 ± 0.21 de | 0.49 ± 0.02 JK | 1.93 ± 0.10 HIJ |
| Fuji | 0.70 ± 0.03 efgh | 3.82 ± 0.16 f | 0.37 ± 0.02 EF | 1.35 ± 0.13 E |
| Indo | 0.56 ± 0.02 d | 3.08 ± 0.15 b | 0.36 ± 0.01 EF | 1.01 ± 0.12 C |
| Ralls Janet | 0.48 ± 0.03 c | 3.17 ± 0.08 bc | 0.34 ± 0.02 EF | 1.70 ± 0.08 F |
| Flavan-3-ols | Dihydrochalcones | Phenolic Acids | Anthocyanins | Flavonols | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | Epi | Phl | CA | C3gal | C3ara | Rut | Q3gal | Q3glc | Q3rha | Total | |
| Peel | |||||||||||
| Summer Prince | 36 ± 1 l | 213 ± 7 cd | 57 ± 5 ab | 121 ± 1 f | 58 ± 3 e | 5 ± 0 bcd | 10 ± 0 cdef | 422 ± 25 j | 101 ± 5 jkl | 151 ± 9 fg | 1174 ± 54 ef |
| Ruby-S | 20 ± 1 h | 166 ± 5 bc | 90 ± 2 fghi | 301 ± 12 k | 223 ± 5 j | 37 ± 1 j | 3 ± 0 ab | 190 ± 6 de | 66 ± 2 cdefg | 73 ± 2 a | 1171 ± 25 ef |
| Summer King | 34 ± 1 l | 81 ± 1 a | 88 ± 2 efgh | 77 ± 1 d | 6 ± 1 a | n.d. | n.d. | 364 ± 9 hi | 103 ± 3 kl | 81 ± 1 a | 834 ± 15 b |
| Tinkerbell | 7 ± 1 ab | 72 ± 3 a | 217 ± 8 m | 1130 ± 12 o | 40 ± 2 cd | n.d. | 12 ± 0 def | 134 ± 5 bc | 52 ± 2 abc | 93 ± 5 ab | 1757 ± 14 j |
| Arisoo | 7 ± 0 ab | 200 ± 19 bc | 81 ± 2 defgh | 50 ± 1 b | 337 ± 35 l | 51 ± 6 h | 35 ± 5 i | 726 ± 70 m | 133 ± 10 m | 256 ± 20 k | 1876 ± 165 j |
| Decobell | 66 ± 6 n | 510 ± 101 k | 338 ± 34 o | 94 ± 8 e | 195 ± 25 i | 14 ± 2 h | 158 ± 13 m | 584 ± 50 l | 512 ± 43 n | 229 ± 21 ij | 2699 ± 302 l |
| Green Ball | 12 ± 0 ef | 161 ± 2 bc | 146 ± 8 k | 48 ± 1 b | n.d. | n.d. | 4 ± 0 abc | 243 ± 21 fg | 85 ± 6 hij | 156 ± 21 g | 855 ± 59 bc |
| Honggeum | 13 ± 0 efg | 424 ± 6 j | 91 ± 2 ghi | 149 ± 2 g | 176 ± 3 h | 27 ± 0 i | 132 ± 4 k | 428 ± 11 j | 107 ± 1 kl | 485 ± 7 m | 2031 ± 21 k |
| Hwangok | 13 ± 1 fg | 266 ± 4 ef | 76 ± 5 cdefg | 30 ± 1 a | n.d. | n.d. | 31 ± 2 hi | 506 ± 9 k | 114 ± 4 l | 345 ± 3 l | 1381 ± 24 ghi |
| Picnic | 9 ± 0 bcd | 277 ± 7 efg | 74 ± 4 cde | 637 ± 13 m | 53 ± 0 de | 7 ± 0 def | 15 ± 0 f | 149 ± 1 c | 60 ± 0 abcde | 121 ± 4 cde | 1400 ± 14 ghi |
| Gala | 23 ± 1 ij | 367 ± 17 i | 47 ± 7 a | 254 ± 6 j | 2 ± 0 a | n.d. | 7 ± 0 abcde | 136 ± 5 bc | 49 ± 2 abc | 113 ± 6 cd | 998 ± 31 cd |
| Sansa | 36 ± 1 l | 262 ± 13 e | 67 ± 1 bcd | 300 ± 10 k | 41 ± 2 cd | 3 ± 0 ab | 15 ± 1 f | 497 ± 29 k | 111 ± 3 l | 107 ± 2 bc | 1437 ± 58 hi |
| Tsugaru | 7 ± 0 ab | 252 ± 2 de | 79 ± 0 cdefg | 50 ± 1 b | 14 ± 0 ab | n.d. | 6 ± 0 abcd | 257 ± 9 fg | 63 ± 1 bcdef | 204 ± 2 h | 933 ± 14 bc |
| Hongro | 27 ± 1 k | 457 ± 66 j | 107 ± 3 j | 261 ± 10 j | 37 ± 5 cd | 7 ± 1 def | 3 ± 1 a | 98 ± 12 b | 44 ± 3 a | 210 ± 26 hi | 1250 ± 107 efg |
| Sekaiichi | 25 ± 1 j | 369 ± 12 i | 254 ± 10 n | 93 ± 3 e | 25 ± 3 bc | 3 ± 0 abc | 21 ± 2 g | 328 ± 19 h | 63 ± 3 bcdef | 124 ± 2 cde | 1304 ± 31 fghi |
| Alps Otome | 44 ± 0 m | 578 ± 35 l | 141 ± 5 k | 848 ± 12 n | 282 ± 9 k | 26 ± 1 i | 9 ± 1 bcdef | 51 ± 1 a | 79 ± 3 fghi | 83 ± 2 a | 2137 ± 67 k |
| Golden Delicious | 6 ± 0 a | 178 ± 1 bc | 104 ± 2 ij | 210 ± 1 i | n.d. | n.d. | 10 ± 1 cdef | 188 ± 5 d | 84 ± 3 ghij | 135 ± 7 ef | 915 ± 17 bc |
| Jonagold | 7 ± 0 a | 327 ± 2 ghi | 97 ± 4 hij | 168 ± 1 h | 15 ± 1 ab | n.d. | 13 ± 0 ef | 228 ± 7 ef | 77 ± 2 efghi | 355 ± 13 l | 1287 ± 25 fgh |
| Jonathan | 11 ± 0 def | 330 ± 21 hi | 191 ± 5 l | 142 ± 1 g | 113 ± 1 g | 8 ± 0 f | 5 ± 0 abc | 271 ± 10 g | 70 ± 2 defgh | 248 ± 7 jk | 1389 ± 35 ghi |
| Kamhong | 21 ± 1 hi | 315 ± 14 fgh | 139 ± 9 k | 63 ± 3 c | 84 ± 5 f | 5 ± 0 cde | 27 ± 1 h | 376 ± 19 i | 114 ± 5 l | 124 ± 6 cde | 1266 ± 62 efg |
| Yoko | 13 ± 0 fg | 290 ± 6 efgh | 74 ± 2 cdef | 46 ± 1 b | 100 ± 5 g | 7 ± 0 ef | 21 ± 2 g | 260 ± 18 fg | 83 ± 5 ghi | 225 ± 8 i | 1120 ± 45 de |
| Fuji | 8 ± 0 abc | 294 ± 10 efgh | 64 ± 3 bc | 264 ± 3 j | 116 ± 4 g | 18 ± 1 h | 147 ± 5 l | 344 ± 8 hi | 91 ± 2 ijk | 114 ± 4 cde | 1461 ± 29 i |
| Indo | 15 ± 1 g | 157 ± 24 b | 78 ± 5 cdefg | 36 ± 4 a | n.d. | n.d. | 14 ± 3 f | 158 ± 21 cd | 53 ± 6 abcd | 159 ± 20 g | 672 ± 87 a |
| Ralls Janet | 10 ± 0 cde | 207 ± 4 bcd | 63 ± 2 bc | 455 ± 5 l | 77 ± 4 f | 4 ± 0 abc | 53 ± 3 j | 142 ± 10 c | 47 ± 2 ab | 130 ± 5 de | 1187 ± 29 ef |
| Pulp | |||||||||||
| Summer Prince | 48 ± 1 K | 121 ± 1 H | 4 ± 0 A | 427 ± 6 K | n.d. | n.d. | n.d. | 6 ± 0 K | 6 ± 0 F | n.d. | 611 ± 6 H |
| Ruby-S | 53 ± 0 L | 6 ± 0 A | n.d. | 652 ± 6 P | n.d. | n.d. | 3 ± 0 A | 8 ± 0 M | n.d. | n.d. | 722 ± 7 J |
| Summer King | 14 ± 0 A | 4 ± 0 A | n.d. | 243 ± 5 F | n.d. | n.d. | n.d. | 6 ± 0 GHIJ | 5 ± 0 C | n.d. | 272 ± 5 BC |
| Tinkerbell | 89 ± 3 P | 4 ± 0 A | n.d. | 1623 ± 3 U | 10 ± 0 A | n.d. | 4 ± 0 B | 6 ± 0 J | 5 ± 0 D | n.d. | 1741 ± 6 O |
| Arisoo | 28 ± 1 DE | 5 ± 0 A | n.d. | 215 ± 3 D | n.d. | n.d. | n.d. | 5 ± 0 DEF | n.d. | n.d. | 253 ± 4 AB |
| Decobell | 53 ± 1 L | 298 ± 2 L | 9 ± 0 L | 268 ± 3 G | n.d. | n.d. | 4 ± 1 B | 7 ± 0 L | n.d. | n.d. | 639 ± 6 I |
| Green Ball | 30 ± 3 FG | 80 ± 10 D | 4 ± 0 B | 142 ± 17 B | n.d. | n.d. | n.d. | 5 ± 0 CDE | 5 ± 0 D | n.d. | 267 ± 30 BC |
| Honggeum | 44 ± 1 J | 100 ± 2 F | 4 ± 0 D | 562 ± 8 N | n.d. | n.d. | n.d. | n.d. | 6 ± 0 E | n.d. | 717 ± 10 J |
| Hwangok | 125 ± 2 Q | 100 ± 1 F | 7 ± 0 K | 231 ± 2 EF | n.d. | n.d. | n.d. | 6 ± 0 IJ | n.d. | n.d. | 469 ± 5 E |
| Picnic | 84 ± 2 O | 83 ± 2 D | 6 ± 0 H | 1183 ± 23 T | n.d. | n.d. | n.d. | 5 ± 0 BCD | 5 ± 0 B | n.d. | 1367 ± 27 M |
| Gala | 29 ± 1 EF | 98 ± 2 EF | 6 ± 0 I | 523 ± 11 M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 657 ± 14 I |
| Sansa | 71 ± 0 N | 113 ± 1 G | 5 ± 0 E | 775 ± 2 R | n.d. | n.d. | n.d. | 6 ± 0 EFG | 5 ± 0 A | n.d. | 974 ± 3 L |
| Tsugaru | 23 ± 0 C | 93 ± 1 E | n.d. | 125 ± 1 A | n.d. | n.d. | n.d. | 5 ± 0 ABC | n.d. | n.d. | 246 ± 1 A |
| Hongro | 70 ± 2 N | 130 ± 2 I | 4 ± 0 C | 755 ± 4 Q | n.d. | n.d. | n.d. | 6 ± 0 HIJ | 6 ± 0 E | n.d. | 971 ± 6 L |
| Sekaiichi | 70 ± 3 N | 140 ± 6 J | 5 ± 0 D | 225 ± 9 DE | n.d. | n.d. | n.d. | 6 ± 0 DEFG | 8 ± 0 H | n.d. | 452 ± 18 E |
| Alps Otome | 29 ± 1 DEF | 221 ± 10 K | 4 ± 0 AB | 1164 ± 13 S | n.d. | n.d. | n.d. | 6 ± 0 IJ | n.d. | n.d. | 1424 ± 24 N |
| Golden Delicious | 19 ± 0 B | 85 ± 1 D | 4 ± 0 AB | 349 ± 1 H | n.d. | n.d. | n.d. | 6 ± 0 FGH | 6 ± 0 G | n.d. | 468 ± 2 E |
| Jonagold | 40 ± 0 I | 98 ± 1 EF | 5 ± 0 F | 394 ± 3 J | n.d. | n.d. | n.d. | 6 ± 0 HIJ | 8 ± 0 I | n.d. | 551 ± 4 G |
| Jonathan | 38 ± 1 H | 141 ± 4 J | 7 ± 0 J | 461 ± 10 L | n.d. | n.d. | n.d. | 7 ± 0 L | n.d. | n.d. | 653 ± 14 I |
| Kamhong | 57 ± 1 M | 48 ± 1 B | 4 ± 0 D | 167 ± 2 C | n.d. | n.d. | n.d. | 6 ± 0 FGHI | n.d. | n.d. | 282 ± 4 C |
| Yoko | 32 ± 0 G | 132 ± 1 I | 5 ± 0 G | 163 ± 1 C | n.d. | n.d. | n.d. | 5 ± 0 A | n.d. | n.d. | 338 ± 3 D |
| Fuji | 19 ± 0 B | 99 ± 2 EF | n.d. | 375 ± 5 I | n.d. | n.d. | n.d. | 5 ± 0 AB | n.d. | n.d. | 498 ± 7 F |
| Indo | 14 ± 0 A | 68 ± 1 C | n.d. | 238 ± 4 EF | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 320 ± 5 D |
| Ralls Janet | 27 ± 1 D | 108 ± 2 G | 4 ± 0 AB | 620 ± 12 O | n.d. | n.d. | n.d. | 5 ± 0 ABC | n.d. | n.d. | 764 ± 15 K |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Ku, K.-H.; Jeong, M.-C.; Kwon, S.-I.; Lee, J. Metabolite Profiling and Antioxidant Activity of 10 New Early- to Mid-Season Apple Cultivars and 14 Traditional Cultivars. Antioxidants 2020, 9, 443. https://doi.org/10.3390/antiox9050443
Kim I, Ku K-H, Jeong M-C, Kwon S-I, Lee J. Metabolite Profiling and Antioxidant Activity of 10 New Early- to Mid-Season Apple Cultivars and 14 Traditional Cultivars. Antioxidants. 2020; 9(5):443. https://doi.org/10.3390/antiox9050443
Chicago/Turabian StyleKim, Inhwan, Kyung-Hyung Ku, Moon-Cheol Jeong, Soon-Il Kwon, and Jihyun Lee. 2020. "Metabolite Profiling and Antioxidant Activity of 10 New Early- to Mid-Season Apple Cultivars and 14 Traditional Cultivars" Antioxidants 9, no. 5: 443. https://doi.org/10.3390/antiox9050443