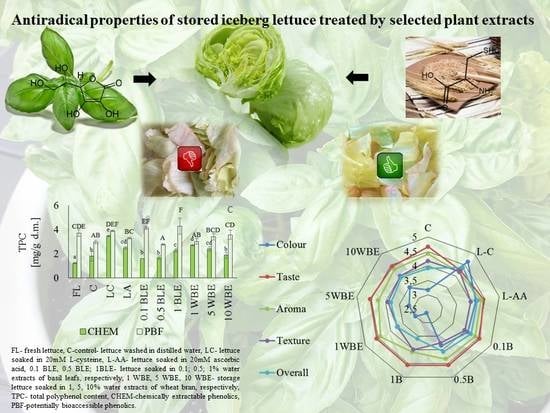
Effect of Basil Leaves and Wheat Bran Water Extracts on Antioxidant Capacity, Sensory Properties and Microbiological Quality of Shredded Iceberg Lettuce during Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Preparation of a Solution of Inhibitors and Lettuce Treatment
2.3. Consumer Evaluation
2.4. Low-Molecular Antioxidant Compounds and Antioxidant Properties
2.4.1. Extraction Procedures
In Vitro Digestion
Chemical Extraction
Ascorbic Acid Assay
2.4.2. Low-Molecular Weight Antioxidants
Phenolic Content (TPC)
Ascorbic Acid Content (l-AA)
2.4.3. Antioxidant Properties
Ability to Quench ABTS•+
Ability to Scavenge DPPH
Reducing Power (RP)
2.5. Microbiological Quality
Total Mesophilic Bacteria
Lactic Acid Bacteria
Molds and Yeasts
Coliforms
2.6. Theoretical Approach
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Augustin, L.S.A.; Nazionale, I.; Irccs, T.; Pascale, F.G. Diet and Cancer. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 471–501. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Heimler, D.; Isolani, L.; Vignolini, P.; Tombelli, S.; Romani, A. Polyphenol content and antioxidant activity in some freshly consumed salads. J. Agric. Food Chem. 2007, 55, 1724–1729. [Google Scholar] [CrossRef]
- Bencardino, D.; Vitali, L.A.; Petrelli, D. Microbiological evaluation of ready-to-eat iceberg lettuce during shelf-life and effectiveness of household washing methods. Ital. J. Food Saf. 2018, 7, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular modeling of peroxidase and polyphenol Oxidase: Substrate specificity and active site comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Bhandari, B.; Jiang, F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. LWT 2019, 113, 108315. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H. Effects of potential organic compatible sanitisers on organic and conventional fresh-cut lettuce (Lactuca sativa Var. Crispa L). Food Control 2017, 72, 20–26. [Google Scholar] [CrossRef]
- Sikora, M.; Złotek, U.; Świeca, M. Effect of basil leaves and wheat bran water extracts on enzymatic browning of shredded storage iceberg lettuce. Int. J. Food Sci. Technol. 2020, 55, 1318–1325. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Liang, X.; Wu, Y.; Qiu, J.; Zhong, K.; Gao, H. A Potent antibrowning agent from pine needles of Cedrus deodara: 2R, 3R -dihydromyricetin. J. Food Sci. 2014, 79, 1643–1648. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Lante, A. Bioresource technology antibrowning potential of Brassicacaea processing water. Bioresour. Technol. 2010, 101, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Moon, K.; Lee, C. Rhubarb juice as a natural antibrowning agent. Food Chem. 2000, 65, 1288–1289. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, F.M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of methodology to analyze ascorbic and dehydroascorbic acid in vegetables. Quim. Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Złotek, U.; Świeca, M. Elicitation effect of Saccharomyces cerevisiae yeast extract on main health-promoting compounds and antioxidant and anti-inflammatory potential of butter lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2016, 96, 2565–2572. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- International Student Organization. Microbiology of the Food Chain–Horizontal Method for the Enumeration of Microorganisms–Part 2: Colony Count at 30 Degrees C by the Surfaceplating Technique; PN EN ISO 4833–2; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- International Student Organization. Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Microorganisms-Colony-Count Technique at 30 °C; PN-ISO 15214; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- International Student Organization. Microbiology of Food and Animal Feeding Stuffs—Horizontalmethod for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique Inproducts with Water Activity Greater than 0, 95; PN-ISO 21527–1; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Student Organization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique; PN-ISO 4832; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Holzwarth, M.; Wittig, J.; Carle, R.; Kammerer, D.R. Influence of putative polyphenoloxidase (PPO) inhibitors on strawberry (Fragaria x ananassa Duch.) PPO, anthocyanin and color stability of stored purees. LWT—Food Sci. Technol. 2013, 52, 116–122. [Google Scholar] [CrossRef]
- Capotorto, I.; Amodio, M.L.; Diaz, M.T.B.; de Chiara, M.L.V.; Colelli, G. Effect of anti-browning solutions on quality of fresh-cut fennel during storage. Postharvest Biol. Technol. 2018, 137, 21–30. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-cysteine as anti-browning agent in fresh-cut lettuce processing. J. Food Process. Preserv. 2015, 39, 985–993. [Google Scholar] [CrossRef]
- Peng, X.; Yang, J.; Cui, P.; Chen, F.; Fu, Y.; Hu, Y.; Zhang, Q.; Xia, X. Influence of allicin on quality and volatile compounds of fresh-cut stem lettuce during cold storage. LWT—Food Sci. Technol. 2015, 60, 300–307. [Google Scholar] [CrossRef]
- Allende, A.; Aguayo, E.; Artés, F. Microbial and sensory quality of commercial fresh processed red lettuce throughout the production chain and shelf life. Int. J. Food Microbiol. 2004, 91, 109–117. [Google Scholar] [CrossRef]
- Yuan, J.P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Altunkaya, A.; Gökmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 4, 1173–1179. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885. [Google Scholar] [CrossRef]
- Lafarga, T.; Villaró, S.; Rivera, A.; Bobo, G.; Aguiló-Aguayo, I. Bioaccessibility of polyphenols and antioxidant capacity of fresh or minimally processed modern or traditional lettuce (Lactuca sativa L.) varieties. J. Food Sci. Technol. 2019, 57, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice- and milk-based beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crop. Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Ferrante, A.; Martinetti, L.; Maggiore, T. Biochemical changes in cut vs. intact lamb’s lettuce (Valerianella olitoria) leaves during storage. Int. J. Food Sci. Technol. 2009, 44, 1050–1056. [Google Scholar] [CrossRef]
- Zhan, L.; Li, Y.; Hu, J.; Pang, L.; Fan, H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Cardinali, A.; D’Antuono, I.; Serio, F. Relationship between quality parameters and the overall appearance in lettuce during storage. Int. J. Food Process. Technol. 2014, 1, 18–26. [Google Scholar] [CrossRef]
- Baur, S.; Klaiber, R.G.; Koblo, A.; Carle, R. Effect of different washing procedures on phenolic metabolism of shredded, packaged iceberg lettuce during storage. J. Agric. Food Chem. 2004, 52, 7017–7025. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Rico, D.; Barry-Ryan, C. Green tea extract as a natural antioxidant to extend the shelf-life of fresh-cut lettuce. Innov. Food Sci. Emerg. Technol. 2008, 9, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, H.B.; Chung, H.S.; Moon, K.D. Browning control of fresh-cut lettuce by phytoncide treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef]
- Peng, X.; Li, R.; Zou, R.; Chen, J.; Zhang, Q.; Cui, P.; Chen, F.; Fu, Y.; Yang, J.; Xia, X. Allicin inhibits microbial growth and oxidative browning of fresh-cut lettuce (Lactuca sativa) during refrigerated storage. Food Bioprocess Technol. 2014, 7, 1597–1605. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gökmen, V. Effect of grape seed extract on phenolic profile and browning of fresh-cut lettuce (l. sativa). J. Food Biochem. 2012, 36, 268–274. [Google Scholar] [CrossRef]
- Allende, A.; McEvoy, J.; Tao, Y.; Luo, Y. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 2009, 20, 230–234. [Google Scholar] [CrossRef]
- Ponce, A.; Roura, S.I.; del Moreira, M.R. Essential oils as biopreservatives: Different methods for the technological application in lettuce leaves. J. Food Sci. 2011, 76, M34–M40. [Google Scholar] [CrossRef] [PubMed]
- Skrinjar, M.; Nemet, N. Antimicrobial effects of spices and herbs essential oils. Acta Period. Technol. 2009, 220, 195–209. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]

| Time of Storage [Days] | Total Polyphenol Content | ||
|---|---|---|---|
| Sample | Chemically Extractable Phenolics | Potentially Bioaccessible Phenolics | |
| 0 | FL | 1.22 ± 0.06 abc | 3.71 ± 0.27 efghi |
| 3 | C | 1.37 ± 0.16 abcd | 3.91 ± 0.07 ghi |
| LC | 2.59 ± 0.15 jkl | 3.83 ± 0.04 fghi | |
| l-AA | 2.11 ± 0.01 ghij | 3.92 ± 0.05 ghi | |
| 0.1 BLE | 1.27 ± 0.05 abc | 3.58 ± 0.03 cdefgh | |
| 0.5 BLE | 1.30 ± 0.12 abc | 2.59 ± 0.67 a | |
| 1 BLE | 1.55 ± 0.30 bcdef | 3.36 ± 0.32 bcdefg | |
| 1 WBE | 1.42 ± 0.04 abcde | 3.19 ± 0.09 abcd | |
| 5 WBE | 1.32 ± 0.14 abcd | 3.04 ± 0.03 abcd | |
| 10 WBE | 1.01 ± 0.17 a | 3.42 ± 0.27 bcdefg | |
| 5 | C | 1.40 ± 0.22 abcde | 2.90 ± 0.02 abc |
| LC | 2.99 ± 0.41 lm | 4.28 ± 0.27 i | |
| l-AA | 1.96 ± 0.18 fghi | 3.60 ± 0.07 defgh | |
| 0.1 BLE | 1.24 ± 0.06 abc | 3.42 ± 0.11 bcdefg | |
| 0.5 BLE | 1.40 ± 0.24 abcde | 3.80 ± 0.13 fghi | |
| 1 BLE | 2.13 ± 0.09 ghij | 3.83 ± 0.03 fghi | |
| 1 WBE | 1.24 ± 0.03 abc | 3.47 ± 0.24 cdefg | |
| 5 WBE | 1.43 ± 0.02 abcde | 3.79 ± 0.17 fghi | |
| 10 WBE | 1.17 ± 0.25 ab | 3.66 ± 0.32 defgh | |
| 8 | C | 1.80 ± 0.41 defgh | 2.99 ± 0.15 abcd |
| LC | 3.47 ± 0.05 m | 3.88 ± 0.06 ghi | |
| AA | 2.48 ± 0.11 jk | 3.29 ± 0.07 bcdefg | |
| 0.1 BLE | 1.59 ± 0.02 bcdef | 4.13 ± 0.12 hi | |
| 0.5 BLE | 1.68 ± 0.15 cdefg | 2.76 ± 0.06 ab | |
| 1 BLE | 2.25 ± 0.12 hijk | 4.28 ± 0.66 i | |
| 1 WBE | 2.71 ± 0.07 kl | 3.02 ± 0.23 abcd | |
| 5 WBE | 2.40 ± 0.19 ijk | 3.42 ± 0.27 bcdefg | |
| 10 WBE | 1.88 ± 0.26 efgh | 3.56 ± 0.38 cdefgh | |
| Time of Storage [Days] | Sample | Total [μg/g d.m.] | Content of Ascorbic Acid [μg/g d.m.] |
|---|---|---|---|
| 0 | FL | 9.06 ± 0.40 efg | 8.65 ± 0.23 jk |
| 3 | C | 9.35 ± 0.05 g | 8.75 ± 0.10 k |
| LC | 8.55 ± 0.02 efg | 8.00 ± 0.08 hi | |
| l-AA | 8.36 ± 0.06 efg | 7.90 ± 0.34 h | |
| 0.1 BLE | 6.60 ± 0.10 d | 5.31 ± 0.05 f | |
| 0.5 BLE | 6.29 ± 0.21 abcd | 5.12 ± 0.11 def | |
| 1 BLE | 6.09 ± 0.09 abcd | 5.08 ± 0.05 def | |
| 1 WBE | 5.45 ± 0.24 abc | 4.41 ± 0.18 ab | |
| 5 WBE | 5.19 ± 0.05 a | 4.17 ± 0.04 a | |
| 10 WBE | 5.58 ± 0.18 abcd | 4.49 ± 0.08 abc | |
| 5 | C | 9.27 ± 0.12 g | 8.48 ± 0.16 ijk |
| LC | 7.97 ± 0.22 e | 7.29 ± 0.17 g | |
| l-AA | 8.76 ± 0.31 efg | 8.13 ± 0.08 hij | |
| 0.1 BLE | 6.46 ± 0.05 cd | 5.26 ± 0.01 ef | |
| 0.5 BLE | 6.56 ± 0.01 cd | 5.28 ± 0.04 f | |
| 1 BLE | 5.97 ± 0.90 abcd | 4.93 ± 0.15 bcdef | |
| 1 WBE | 5.65 ± 0.50 abcd | 4.67 ± 0.06 abcd | |
| 5 WBE | 5.58 ± 0.52 ab | 4.71 ± 0.12 abcde | |
| 10 WBE | 5.33 ± 0.70 ab | 4.45 ± 0.10 abc | |
| 8 | C | 8.00 ± 0.23 ef | 7.16 ± 0.15 g |
| LC | 8.81 ± 0.52 efg | 7.88 ± 0.33 h | |
| AA | 9.18 ± 0.21 fg | 8.31 ± 0.10 hijk | |
| 0.1 BLE | 5.84 ± 0.10 abcd | 5.23 ± 0.31 def | |
| 0.5 BLE | 5.49 ± 0.42 abcd | 4.92 ± 0.10 bcdef | |
| 1 BLE | 5.59 ± 0.20 abcd | 4.50 ± 0.42 abc | |
| 1 WBE | 5.32 ± 0.11 ab | 4.45 ± 0.22 abc | |
| 5 WBE | 6.11 ± 0.35 abcd | 4.98 ± 0.20 cdef | |
| 10 WBE | 6.37 ± 0.61 bcd | 5.06 ± 0.10 def |
| Time of Storage [Days] | Sample | Ability to Quench ABTS•+ [mg TE/g d.m.] | Ability to Neutralize DPPH• [mg TE/g d.m.] | Reducing Properties [mg TE/g d.m.] | |||
|---|---|---|---|---|---|---|---|
| CHEM | PBF | CHEM | PBF | CHEM | PBF | ||
| 0 | FL | 0.87 ± 0.11 a | 1.39 ± 0.03 a | 1.16 ± 0.07 ab | 1.81 ± 0.15 gh | 9.70 ± 0.15 a | 5.62 ± 0.13 a |
| 3 | C | 0.92 ± 0.04 a | 1.72 ± 0.03 abc | 1.40 ± 0.04 fghi | 2.00 ± 0.08 hij | 9.78 ± 0.24 a | 5.58 ± 0.01 a |
| LC | 2.44 ± 0.06 jk | 2.16 ± 0.09 cdefgh | 1.44 ± 0.02 fghijkl | 1.67 ± 0.03 efg | 28.24 ± 1.03 l | 6.44 ± 0.21 b | |
| l-AA | 1.85 ± 0.25 de | 1.96 ± 0.13 bcdefg | 1.45 ± 0.04 fghijkl | 1.81 ± 0.25 gh | 28.12 ± 0.27 l | 11.33 ± 0.18 f | |
| 0.1BLE | 1.57 ± 0.08 bc | 1.65 ± 0.03 ab | 1.26 ± 0.04 bcde | 2.35 ± 0.08 kl | 18.38 ± 0.02 c | 12.85 ± 0.09 h | |
| 0.5BLE | 1.60 ± 0.11 bc | 1.91 ± 0.25 bcdef | 1.44 ± 0.14 fghijkl | 1.34 ± 0.09 a | 20.05 ± 0.26 d | 12.98 ± 0.34 h | |
| 1 BLE | 1.66 ± 0.01 cd | 2.03 ± 0.13 bcdefgh | 1.41 ± 0.02 fghij | 1.54 ± 0.04 abcdef | 21.63 ± 0.14 fg | 10.78 ± 0.15 e | |
| 1WBE | 1.99 ± 0.02 ef | 1.77 ± 0.32 abcde | 1.41 ± 0.06 fghij | 1.39 ± 0.03 abcd | 21.52 ± 0.18 fg | 9.04 ± 0.32 c | |
| 5WBE | 1.42 ± 0.08 b | 1.73 ± 0.06 abcde | 1.10 ± 0.02 a | 1.38 ± 0.06 abcd | 16.69 ± 0.21 b | 13.48 ± 0.03 i | |
| 10WBE | 1.57 ± 0.01 bc | 2.39 ± 0.16 ghij | 1.54 ± 0.04 jklmn | 1.78 ± 0.05 fgh | 19.98 ± 1.07 d | 19.28 ± 0.25 o | |
| 5 | C | 1.60 ± 0.48 bc | 2.19 ± 0.40 defgh | 1.35 ± 0.23 defg | 2.35 ± 0.03 kl | 20.43 ± 0.61 de | 10.15 ± 0.02 d |
| LC | 3.35 ± 0.47 n | 2.83 ± 0.42 jkl | 1.64 ± 0.24 n | 2.25 ± 0.25 jk | 27.78 ± 0.68 l | 11.55 ± 0.13 f | |
| l-AA | 2.19 ± 0.40 fgh | 2.05 ± 0.41 bcdefgh | 1.23 ± 0.25 abcd | 1.41 ± 0.10 abcd | 24.48 ± 0.01 h | 16.65 ±0.20 m | |
| 0.1BLE | 1.84 ± 0.42 de | 2.06 ± 0.42 bcdefgh | 1.20 ± 0.25 abc | 1.97 ± 0.23 hi | 18.94 ± 0.12 c | 13.47 ± 0.27 i | |
| 0.5BLE | 2.24 ± 0.42 hij | 2.73 ± 0.44 ikl | 1.32 ± 0.24 cdef | 1.65 ± 0.10 defg | 26.74 ± 0.72 jk | 13.57 ± 0.04 i | |
| 1 BLE | 2.51 ± 0.44 k | 2.10 ± 0.40 bcdefgh | 1.38 ± 0.24 efgh | 2.16 ± 0.25 ijk | 25.54 ± 0.08 i | 15.03 ± 0.16 l | |
| 1WBE | 2.02 ± 0.44 efg | 2.19 ± 0.30 defgh | 1.97 ± 0.25 o | 1.75 ± 0.16 fgh | 25.08 ± 0.22 hi | 12.05 ± 0.49 g | |
| 5WBE | 2.03 ± 0.46 efgh | 2.40 ± 0.27 ghij | 1.47 ± 0.06 ghijkl | 1.35 ± 0.08 ab | 21.08 ± 0.46 ef | 13.67 ± 0.15 i | |
| 10WBE | 1.82 ± 0.48 de | 2.71 ± 0.21 ijkl | 1.44 ± 0.01 fghijkl | 1.33 ± 0.04 a | 22.14 ± 0.26 g | 18.53 ± 0.16 n | |
| 8 | C | 2.22 ± 0.13 ghi | 2.31 ± 0.02 fghi | 1.56 ± 0.10 lmn | 1.83 ± 0.08 gh | 28.09 ± 0.16 l | 16.2 ± 0.15 m |
| LC | 3.76 ± 0.22 o | 2.89 ± 0.21 kl | 1.42 ± 0.02 fghijk | 2.00 ± 0.03 hij | 27.61 ± 0.06 kl | 18.15 ± 0.02 n | |
| l-AA | 2.80 ± 0.06 lm | 2.15 ± 0.20 cdefgh | 1.38 ± 0.06 efgh | 1.38 ± 0.36 abc | 18.02 ± 0.16 c | 23.6 ± 0.27 p | |
| 0.1BLE | 2.43 ± 0.22 ijk | 2.47 ± 0.47 hijk | 1.40 ± 0.10 fghi | 2.18 ± 0.08 ijk | 29.76 ± 0.61 m | 14.3 ± 0.08 j | |
| 0.5BLE | 2.78 ± 0.01 lm | 3.17 ± 0.20 i | 1.61 ± 0.12 mn | 1.43 ± 0.05 abcde | 33.21 ± 0. 05 n | 16.6 ± 0.03 m | |
| 1 BLE | 2.86 ± 0.22 m | 2.23 ± 0.12 efgh | 1.36 ± 0.02 defg | 1.63 ± 0.04 cdefg | 29.70 ± 0.33 m | 14.9 ± 0.23 kl | |
| 1WBE | 2.59 ± 0.10 kl | 2.40 ± 0.03 ghij | 1.52 ± 0.05 ijklmn | 1.60 ± 0.02 bcefg | 26.58 ± 0.23 j | 12.4 ± 0.42 g | |
| 5WBE | 2.58 ± 0.01 kl | 2.38 ± 0.18 ghij | 1.55 ± 0.14 klmn | 1.31 ± 0.03 a | 34.56 ± 0.76 o | 14.5 ± 0.02 jk | |
| 10WBE | 2.42 ± 0.02 ijk | 1.82 ± 0.03 abcde | 1.51 ± 0.03 hijklm | 2.52 ± 0.25 l | 24.65 ± 0.95 hi | 14.6 ± 0.42 jkl | |
| Time of Storage [Days] | Sample | RBF | REFabts | REFdpph | REFrp |
|---|---|---|---|---|---|
| FL | 3.04 | 1.60 | 1.55 | 0.58 | |
| 3 | C | 2.86 | 1.87 | 1.43 | 0.57 |
| LC | 1.48 | 0.89 | 1.16 | 0.23 | |
| l-AA | 1.86 | 1.06 | 1.25 | 0.40 | |
| 0.1 BLE | 2.82 | 1.05 | 1.87 | 0.70 | |
| 0.5 BLE | 2.00 | 1.19 | 0.93 | 0.65 | |
| 1 BLE | 2.16 | 1.23 | 1.09 | 0.50 | |
| 1 WBE | 2.24 | 1.04 | 1.03 | 0.42 | |
| 5 WBE | 2.31 | 1.22 | 1.25 | 0.81 | |
| 10 WBE | 3.39 | 1.52 | 1.16 | 0.96 | |
| 5 | C | 2.07 | 1.37 | 1.74 | 0.50 |
| LC | 1.43 | 0.85 | 1.37 | 0.42 | |
| l-AA | 1.84 | 0.94 | 1.14 | 0.68 | |
| 0.1 BLE | 2.75 | 1.12 | 1.65 | 0.71 | |
| 0.5 BLE | 2.70 | 1.22 | 1.25 | 0.51 | |
| 1 BLE | 1.80 | 0.84 | 1.57 | 0.59 | |
| 1 WBE | 2.79 | 1.09 | 0.88 | 0.48 | |
| 5 WBE | 2.65 | 1.18 | 0.92 | 0.65 | |
| 10 WBE | 3.13 | 1.49 | 0.92 | 0.84 | |
| 8 | C | 1.66 | 1.04 | 1.35 | 0.58 |
| LC | 1.12 | 0.77 | 1.45 | 0.66 | |
| l-AA | 1.33 | 0.77 | 0.99 | 1.31 | |
| 0.1 BLE | 2.59 | 1.02 | 1.54 | 0.48 | |
| 0.5 BLE | 2.70 | 1.14 | 1.11 | 0.50 | |
| 1 BLE | 1.91 | 0.78 | 0.95 | 0.50 | |
| 1 WBE | 1.11 | 0.93 | 1.03 | 0.47 | |
| 5 WBE | 1.42 | 0.92 | 0.84 | 0.42 | |
| 10 WBE | 1.89 | 0.75 | 1.56 | 0.59 |
| Time of Storage [Days] | Count of Microorganisms [Log10 CFU/g f.m.] | ||||
|---|---|---|---|---|---|
| Sample | Total Mesophilic Bacteria | Coliforms | Molds and Yeasts | Lactic bacteria | |
| 0 | FL | 4.43 a | 1.91 ab | n.d. | n.d. |
| 5 | C | 5.59 b | 2.51 ef | n.d. | n.d. |
| LC | 6.41 f | 3.42 i | n.d. | n.d. | |
| l-AA | 5.63 b | 1.98 ab | n.d. | n.d. | |
| 0.1 BLE | 5.44 ab | 1.70 a | n.d. | n.d. | |
| 0.5 BLE | 5.44 ab | 2.33 bcde | n.d. | n.d. | |
| 1 BLE | 5.63 b | 2.59 fg | n.d. | n.d. | |
| 1 WBE | 5.55 b | 2.44 def | n.d. | n.d. | |
| 5 WBE | 5.64 b | 2.41 cdef | n.d. | n.d. | |
| 10 WBE | 6.04 c | 2.41 cdef | n.d. | n.d. | |
| 8 | C | 6.38 f | 2.67 g | n.d. | n.d. |
| LC | 7.00 h | 3.33 h | n.d. | n.d. | |
| AA | 6.22 d | 2.71 g | n.d. | n.d. | |
| 0.1 BLE | 6.13 c | 2.13 abc | n.d. | n.d. | |
| 0.5 BLE | 6.09 c | 1.86 a | n.d. | n.d. | |
| 1 BLE | 6.04 c | 2.15 abcd | n.d. | n.d. | |
| 1 WBE | 6.04 c | 2.07 abc | n.d. | n.d. | |
| 5 WBE | 6.33 e | 2.16 abcd | n.d. | n.d. | |
| 10 WBE | 6.77 g | 1.74 a | n.d. | n.d. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, M.; Złotek, U.; Kordowska-Wiater, M.; Świeca, M. Effect of Basil Leaves and Wheat Bran Water Extracts on Antioxidant Capacity, Sensory Properties and Microbiological Quality of Shredded Iceberg Lettuce during Storage. Antioxidants 2020, 9, 355. https://doi.org/10.3390/antiox9040355
Sikora M, Złotek U, Kordowska-Wiater M, Świeca M. Effect of Basil Leaves and Wheat Bran Water Extracts on Antioxidant Capacity, Sensory Properties and Microbiological Quality of Shredded Iceberg Lettuce during Storage. Antioxidants. 2020; 9(4):355. https://doi.org/10.3390/antiox9040355
Chicago/Turabian StyleSikora, Małgorzata, Urszula Złotek, Monika Kordowska-Wiater, and Michał Świeca. 2020. "Effect of Basil Leaves and Wheat Bran Water Extracts on Antioxidant Capacity, Sensory Properties and Microbiological Quality of Shredded Iceberg Lettuce during Storage" Antioxidants 9, no. 4: 355. https://doi.org/10.3390/antiox9040355