Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis
Abstract
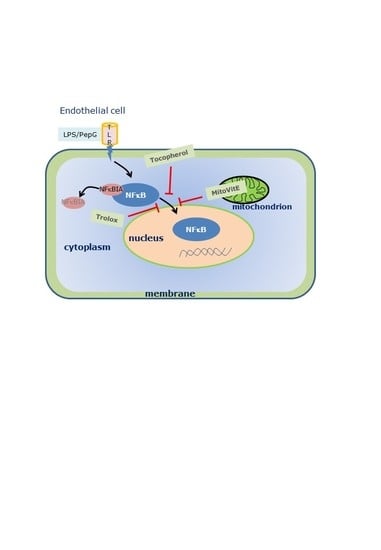
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Oxidative Stress
2.4. Mitochondrial Function
2.5. Differential Gene Expression
2.6. Protein Expression
2.7. Statistical Analysis
3. Results
3.1. Cell Viability
3.1.1. Oxidative Stress
3.1.2. Mitochondrial Function
3.1.3. Gene Expression
3.1.4. Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G. Gram-positive and gram-negative bacterial toxins in sepsis. Virulence 2014, 5, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouser, E.D. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 2004, 4, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 2, 6–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jameson, V.J.; Cochemé, H.M.; Logan, A.; Hanton, L.R.; Smith, R.A.; Murphy, M.P. Synthesis of triphenylphosphonium vitamin E derivatives as mitochondria-targeted antioxidants. Tetrahedron 2015, 71, 8444–8453. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.W.; Hashimoto, N.; Wu, J.; Carey, D.; Li, R.K.; Mickle, D.A.; Weisel, R.D. The cytoprotective effect of Trolox demonstrated with three types of human cells. Biochem. Cell Biol. 1990, 68, 1189–1194. [Google Scholar] [CrossRef]
- Guo, C.; He, Z.; Wen, L.; Zhu, L.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Yuan, H. Cytoprotective effect of Trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biol. Int. 2012, 36, 183–188. [Google Scholar] [CrossRef]
- Hamad, I.; Arda, N.; Pekmez, M.; Karaer, S.; Temizkan, G. Intracellular scavenging activity of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) in the fission yeast, Schizosaccharomyces pombe. J. Nat. Sci. Biol. Med. 2010, 1, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Galley, H.F. Bench to bedside review: Targeting antioxidants to mitochondria in sepsis. Crit. Care 2010, 14, 230. [Google Scholar] [CrossRef] [Green Version]
- Victor, V.M.; Espulgues, J.V.; Hernandez-Mijares, A.; Rocha, M. Oxidative stress and mitochondrial dysfunction in sepsis: A potential therapy with mitochondria-targeted antioxidants. Infect. Disord. Drug Targets 2009, 9, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.A.; Galley, H.F. The relative roles of mitochondrial thioredoxin and glutathione in protecting against mitochondrial dysfunction in an endothelial cell model of sepsis. Biochem. J. 2011, 436, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, D.A.; Almawash, A.M.; Webster, N.R.; Reid, V.; Galley, H.F. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br. J. Anaesth. 2011, 107, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCreath, G.; Scullion, M.M.F.; Lowes, D.A.; Webster, N.R.; Galley, H.F. Pharmacological activation of endogenous protective pathways against oxidative stress under conditions of sepsis. Br. J. Anaesth. 2016, 116, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.T.; Sinai, P.; Kain, S.R. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal. Biochem. 1996, 241, 103–108. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pagé, B.; Pagé, M.; Noël, L.C. A new fluorometric assay for cytotoxicity measurements in vitro. Int. J. Oncol. 2003, 3, 473–476. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, H.; Wang, P.; Liu, H.; Sun, X. Transcription factor NF-kappa B represses ANT1 transcription and leads to mitochondrial dysfunctions. Sci. Rep. 2017, 7, 44708. [Google Scholar] [CrossRef] [Green Version]
- Asehnoune, K.; Strassheim, D.; Mitra, S.; Kim, J.Y.; Abraham, E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappB. J. Immunol. 2004, 172, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Silva, E.; Baggio-Zappia, G.L.; Brunialti, M.K.; Nucci, L.A.; Rigato, O., Jr.; da Silva, I.D.; Machado, F.R.; Salomao, R. Patterns of gene expression in peripheral blood mononuclear cells and outcomes from patients with sepsis secondary to community acquired pneumonia. PLoS ONE 2014, 9, e91886. [Google Scholar] [CrossRef]
- Talwar, S.; Munson, P.J.; Barb, J.; Fiuza, C.; Cintron, A.P.; Logun, C.; Tropea, M.; Khan, S.; Reda, D.; Shelhamer, J.H.; et al. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol. Genom. 2006, 25, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.; Lowes, D.A.; Webster, N.R.; Tahib, J.; Hall, L.; Davies, M.; Beattie, J.H.; Galley, H.F. Low zinc and selenium levels in sepsis are associated with oxidative damage and inflammation. Br. J. Anaesth. 2015, 114, 990–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.U.; Lehmann, L.E.; Schewe, J.C.; Thiele, J.T.; Schröder, S.; Book, M.; Hoeft, A.; Stüber, F. Low serum alpha-tocopherol and selenium are associated with accelerated apoptosis in severe sepsis. Biofactors 2008, 33, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Domínguez-Rodriguez, A.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Ferreres, J.; Cabrera, J.; Igeño, J.C.; et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit. Care 2013, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Daga, M.K.; Khan, N.A.; Singh, H.; Chhoda, A.; Mattoo, S.; Gupta, B.K. Markers of oxidative stress and clinical outcome in critically ill septic patients: A preliminary study from North India. J. Clin. Diagn. Res. 2016, 10, OC35–OC38. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.; Neuzil, J.; Zingg, J.M.; Azzi, S.A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Vitamin E: The shrew waiting to be tamed. Free Radic. Biol. Med. 2009, 46, 543–554. [Google Scholar] [CrossRef]
- Godbout, J.P.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. α-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J. Neuroimmunol. 2004, 149, 101–109. [Google Scholar] [CrossRef]
- Ng, L.T.; Ko, H.J. Comparative effects of tocotrienol-rich fraction, α-tocopherol and α-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012, 134, 920–925. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef]
- McCormick, B.; Lowes, D.A.; Colvin, L.; Torsney, C.; Galley, H.F. Mitochondria targeted vitamin E protects against paclitaxel-induced damage in dorsal root ganglion cells and neuropathic pain in vivo. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, D.A.; Thottakam, B.M.; Webster, N.R.; Murphy, M.P.; Galley, H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008, 45, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, R.J.; Harper, M. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Xu, D.; Farmer, A.; Collett, G.; Grishin, N.V.; Chook, Y.M. Sequence and structural analyses of nuclear export signals in the NESdb database. Mol. Biol. Cell 2012, 23, 3677–3693. [Google Scholar] [CrossRef]
- Kashyap, T.; Argueta, C.; Aboukameel, A.; Unger, T.J.; Klebanov, B.; Mohammad, R.M.; Muqbil, I.; Azmi, A.S.; Drolen, C.; Senapedis, W.; et al. Selinexor, a Selective Inhibitor of Nuclear Export (SINE) compound, acts through NF-κB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget 2016, 7, 78883–78895. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.C.; Choi, S. Recent progress in the molecular recognition and therapeutic Importance of interleukin-1 receptor-associated kinase 4. Molecules 2016, 21, 1529. [Google Scholar] [CrossRef] [Green Version]
- Godbout, J.P.; Berg, B.M.; Krzyszton, C.; Johnson, R.W. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J. Neuroimmunol. 2005, 169, 97–105. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, K.H.; Chung, E.Y.; Chang, Y.S.; Lee, H.; Lee, C.K.; Min, K.R.; Kim, Y. Inhibitory effect of chroman carboxamide on interleukin-6 expression in response to lipopolysaccharide by preventing nuclear factor-kappaB activation in macrophages. Eur. J. Pharmacol. 2006, 543, 158–165. [Google Scholar] [CrossRef]
- Hughes, G.; Murphy, M.P.; Ledgerwood, E.C. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: Evidence from mitochondria-targeted antioxidants. Biochem. J. 2005, 389, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Zang, Q.S.; Sadek, H.; Maass, D.L.; Martinez, B.; Ma, L.; Kilgore, J.A.; Williams, N.S.; Frantz, D.E.; Wigginton, J.G.; Nwariaku, F.E.; et al. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1847–H1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D.E.; Lee, C.-K. What does Stat3 do? J. Clin. Investig. 2002, 109, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.A.; Hyun, M.; Cantwell, M.; Raza, A.; Mertens, C.; Raje, V.; Sisler, J.; Tracy, E.; Torres-Odio, S.; Gispert, S.; et al. Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production. Sci. Signal. 2017, 10, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zingg, J.M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef]
- Ruysschaert, J.M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta 2015, 1848, 1860–1867. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kwiatkowska, K. Modification of pro-inflammatory signaling by dietary components: The plasma membrane as a target. BioEssays 2015, 37, 789–801. [Google Scholar] [CrossRef]





| Gene Name. | LPS/PepG a | LPS/PepG | LPS/PepG | LPS/PepG |
|---|---|---|---|---|
| + MitoVitE b | + α-Tocopherol b | +Trolox b | ||
| GUSB | −2.5 | |||
| (0.27, 0.52) | ||||
| p = 0.05 | ||||
| IĸBKB | −2.5 | |||
| 0.25, 0.56 | ||||
| p = 0.017 | ||||
| IRAK4 | −2.2 | |||
| (1.05, 3.42) | ||||
| p = 0.02 | ||||
| MYD88 | −2.4 | |||
| (0.23, 0.61) | ||||
| p = 0.05 | ||||
| NFκB1 | +4.4 | −4.5 | −3.9 | |
| (0.06, 0.40) | (0.17, 0.28) | (0.18, 0.51) | ||
| p = 0.01 | p = 0.0003 | p = 0.002 | ||
| NFĸBIA | +11.7 | -2.7 | ||
| (0.00001, 0.18) 0.18) | (0.18, 0.56) | |||
| p = 0.02 | p = 0.04 | |||
| PPAR-α | −3.0 | |||
| (0.17, 0.50) | ||||
| p = 0.02 | ||||
| PTGS2 | -3.5 | +3.0 | +2.9 | |
| (0.12, 0.45) | (1.29, 4.62) | (1.30, 4.50) | ||
| p = 0.02 | p = 0.005 | p = 0.006 | ||
| RIPK2 | −5.4 | |||
| (0.12, 0.25) | ||||
| p = 0.001 | ||||
| STAT1 | −3.7 | |||
| (0.20, 0.34) | ||||
| p = 0.0002 | ||||
| STAT3 | −3.1 | |||
| (0.25, 0.40) | ||||
| p = 0.0002 | ||||
| TAB1 | −2.8 | |||
| (0.23, 0.49) | ||||
| p = 0.009 | ||||
| TRAF6 | −2.9 | |||
| (0.24, 0.44) | ||||
| p = 0.0003 | ||||
| XPO1 | −2.7 | |||
| (0.98, 4.33) | ||||
| p = 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minter, B.E.; Lowes, D.A.; Webster, N.R.; Galley, H.F. Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis. Antioxidants 2020, 9, 195. https://doi.org/10.3390/antiox9030195
Minter BE, Lowes DA, Webster NR, Galley HF. Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis. Antioxidants. 2020; 9(3):195. https://doi.org/10.3390/antiox9030195
Chicago/Turabian StyleMinter, Beverley E., Damon A. Lowes, Nigel R. Webster, and Helen F. Galley. 2020. "Differential Effects of MitoVitE, α-Tocopherol and Trolox on Oxidative Stress, Mitochondrial Function and Inflammatory Signalling Pathways in Endothelial Cells Cultured under Conditions Mimicking Sepsis" Antioxidants 9, no. 3: 195. https://doi.org/10.3390/antiox9030195