Effect of Ultrasound-Treated Arabinoxylans on the Oxidative Stability of Soybean Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ultrasonic Treatment of AX
2.2.2. Physicochemical Properties
2.2.3. Fourier Transform Infrared Spectroscopy Analysis
2.2.4. Ferulic Acid Content
2.2.5. Total Phenolic Content
2.2.6. Radical Scavenging Capacity
2.2.7. Oxidative Stability of Oils by Rancimat Method
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Ultrasonic Treatment on the Macromolecular Features of AX
3.2. FT-IR Analysis
3.3. Total Phenolic Content and Antioxidant Capacity
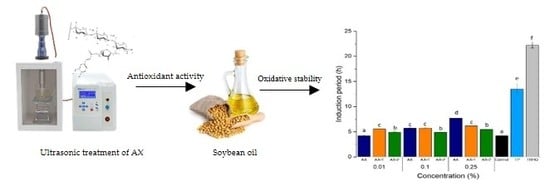
3.4. Oxidative Stability of Soybean Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.; Segovia, F.; Skowyra, M.; Almajano, M. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Pro-oxidant effects of b-carotene during thermal oxidation of edible oils. J. Am. Oil Chem. Soc. 2013, 90, 881–889. [Google Scholar] [CrossRef]
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Asnaashari, M.; Tajik, R.; Haddad Khodaparast, M.H. Antioxidant activity of raspberry (Rubus fruticosus) leaves extract and its effect on oxidative stability of sunflower oil. J. Food Sci. Technol. 2015, 52, 5180–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohdaly, A.A.; Sarhan, M.A.; Mahmoud, A.; Ramadan, M.F.; Smetanska, I. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010, 123, 1019–1026. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascón-Chu, A.; Astiazarán-García, H.; Valencia-Rivera, D.E.; Brown-Bojorquez, F.; Alday, E.; Velazquez, C. Arabinoxylan-based particles: In vitro antioxidant and cytotoxicity on a human colon cell line. Medicina 2019, 55, 349. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Yadav, M.P.; López-Franco, Y.L.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Brown-Bojorquez, F.; Silva-Campa, E.; Pedroza-Montero, M. Partial removal of protein associated with arabinoxylans: Impact on the viscoelasticity, crosslinking content, and microstructure of the gels formed. J. Appl. Polym. Sci. 2019, 136, 47300. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Arabinoxylans: Techonologically and nutrionally functional plant polysaccharides. In Functional Food Carbohydrates; Biliaderis, C.G., Izydorczyk, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 249–283. [Google Scholar]
- Marquez-Escalante, J.A.; Carvajal-Millan, E. Feruloylated Arabinoxylans from Maize Distiller’s Dried Grains with Solubles: Effect of Feruloyl Esterase on their Macromolecular Characteristics, Gelling, and Antioxidant Properties. Sustainability 2019, 11, 6449. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Balandrano, D.D.; Báez-González, J.G.; Carvajal-Millán, E.; Muy-Rangel, D.; Urías-Orona, V.; Martínez-López, A.L.; Márquez-Escalante, J.A.; Heredia, J.B.; Beta, T.; Niño-Medina, G. Alkali-extracted feruloylated arabinoxylans from nixtamalized maize bran byproduct: A synonymous with soluble antioxidant dietary fiber. Waste Biomass Valorization 2018. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated arabinoxylans and their gels: Functional properties and potential application as antioxidant and anticancer agent. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez-Escalante, J.; Carvajal-Millan, E.; López-Franco, Y.L.; Valenzuela-Soto, E.M. Prebiotic effect of Arabinoxylans and Arabinoxylan-Oligosaccharides and the relationship with good health promotion. CienciaUAT 2018, 13, 146–164. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H.; Ara, G.; Ara, X.; Ara, A. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Vargas-Albores, F.; Fierro-Islas, J.M.; Gollas-Galván, T.; Magdaleno-Moncayo, D.; Rascon-Chu, A.; Martínez-Porchas, M.; Lago-Lestón, A. Arabinoxylans and gelled arabinoxylans used as anti-obesogenic agents could protect the stability of intestinal microbiota of rats consuming high-fat diets. Int. J. Food Sci. Nutr. 2019, 71, 74–83. [Google Scholar] [CrossRef]
- Yan, J.; Jia, X.; Feng, L.; Yadav, M.; Li, X.; Yin, L. Rheological and emulsifying properties of arabinoxylans from various cereal brans. J. Cereal Sci. 2019, 90, 102844. [Google Scholar] [CrossRef]
- Yadav, M.P.; Johnston, D.B.; Hotchkiss, A.T.; Hicks, K.B. Corn fiber gum: A potential gum arabic replacer for beverage flavor emulsification. Food Hydrocoll. 2007, 21, 1022–1030. [Google Scholar] [CrossRef]
- Li, L.; Ma, S.; Fan, L.; Zhang, C.; Pu, X.; Zheng, X.; Wang, X. The influence of ultrasonic modification on arabinoxylans properties obtained from wheat bran. Int. J. Food Sci. Technol. 2016, 51, 2338–2344. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Antioxidant capacity of arabinoxylan oligosaccharide fractions prepared from wheat aleurone using Trichoderma viride or Neocallimastix patriciarum xylanase. Food Chem. 2015, 167, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors in fl uencing their antioxidant capacity. Bioact. Carbohydr. Diet. Fibre 2017, 10, 20–26. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Saulnier, L. Experimental evidence for a semi-flexible conformation. Carbohydr. Res. 2001, 330, 365–372. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Rouau, X.; Chaynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudeu, E.; Montero, J.L.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin—Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Antioxidant capacity of water extractable arabinoxylan from commercial barley, wheat and wheat fractions. Cereal Chem. 2015, 92, 29–36. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; He, R. Effect of ultrasonic degradation on in vitro antioxidant activity of polysaccharides from Porphyra yezoensis (Rhodophyta). Food Sci. Technol. Int. 2008, 14, 479. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, W.; Liang, S.; Zhang, H.; Yang, H.; Li, M. Effects of ultrasonic treatment on the molecular weight and anti-inflammatory activity of oxidized konjac glucomannan. CyTA J. Food 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Cheung, Y.; Leung, P.; Wu, J. Ultrasonic treatment for improved solution properties of a high-molecular weight exopolysaccharide produced by a medicinal fungus. Bioresour. Technol. 2010, 101, 5517–5522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hromádková, Z.; Smestad, B.; Polovka, M.; Koˇ, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Urias-Orona, V.; Huerta-Oros, J.; Carvajal-Millán, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; Gardea, A.A. Component analysis and free radicals scavenging activity of Cicer arietinum L. husk pectin. Molecules 2010, 15, 6948–6955. [Google Scholar] [CrossRef] [Green Version]
- Egüés, I.; Stepan, A.M.; Eceiza, A.; Toriz, G.; Gatenholm, P.; Labidi, J. Corncob arabinoxylan for new materials. Carbohydr. Polym. 2014, 102, 12–20. [Google Scholar] [CrossRef]
- Kacurakova, M.; Wellner, N.; Ebringerova, A.; Hromadkova, Z.; Wilson, R.H.; Belton, P.S. Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocoll. 1999, 13, 35–41. [Google Scholar] [CrossRef]
- Rouau, X.S.L.G.F.S.P. Plant cell wall polysaccharides in storage organs: Xylans (Food Applications). In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier Inc.: Waltham, MA, USA, 2013; pp. 1–32. ISBN 9780124095472. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, A.M.T.M.; Medeiros, M.L.; Silva, M.A.A.D.; Silva, I.A.A.; Soledade, L.E.B.; Souza, A.L.; Queiroz, N.; Souza, A.G. Rancimat and PDSC accelerated techniques for evaluation of oxidative stability of soybean oil with plant extracts. J. Therm. Anal. Calorim. 2013, 114, 827–832. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of Low-Density Lipoprotein (LDL) cholesterol oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]




| Sample | Mw | PI | [η] | FA 1 |
|---|---|---|---|---|
| kDa | dL/g | µg/mg AX | ||
| AX | 598 | 1.82 | 13 | 6.72 ± 0.07 |
| AX-1 | 476 | 1.85 | 11 | 6.49 ± 0.01 |
| AX-2 | 744 | 1.75 | 12 | 6.24 ± 0.28 |
| Sample | EC50 | Antioxidant Capacity | Total Phenolic |
|---|---|---|---|
| µg/mL | µmol TEAC/g AX | mg EGA/g | |
| AX | 332.96 ± 4.08 a | 32.09 ± 4.77 c | 11.67 ± 0.37 a |
| AX-1 | 225.17 ± 3.05 c | 44.30 ± 2.27 a | 11.84 ± 0.53 a |
| AX-2 | 270.99 ± 8.75 b | 39.59 ± 0.24 b | 12.20 ± 0.24 a |
| Treatment | PF 1 | ||
|---|---|---|---|
| Concentration (% w/v) | |||
| 0.01 | 0.10 | 0.25 | |
| AX | 1.01 ± 0.02 a | 1.33 ± 0.02 b | 1.83 ± 0.03 c |
| AX-1 | 1.31 ± 0.01 c | 1.35 ± 0.02 b | 1.46 ± 0.00 b |
| AX-2 | 1.17 ± 0.01 b | 1.15 ± 0.01 a | 1.29 ± 0.05 a |
| 0.02% (w/v) TBHQ 2 | 5.24 ± 0.52 e | ||
| 0.02% (w/v) Tocopherol | 3.18 ± 1.10 d | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Encinas, M.A.; Carvajal-Millan, E.; Ortega-García, J.; Santiago-Gómez, L.B.; De Anda-Flores, Y.; Martínez-Robinson, K.G.; Valencia-Rivera, D.E. Effect of Ultrasound-Treated Arabinoxylans on the Oxidative Stability of Soybean Oil. Antioxidants 2020, 9, 147. https://doi.org/10.3390/antiox9020147
Mendez-Encinas MA, Carvajal-Millan E, Ortega-García J, Santiago-Gómez LB, De Anda-Flores Y, Martínez-Robinson KG, Valencia-Rivera DE. Effect of Ultrasound-Treated Arabinoxylans on the Oxidative Stability of Soybean Oil. Antioxidants. 2020; 9(2):147. https://doi.org/10.3390/antiox9020147
Chicago/Turabian StyleMendez-Encinas, Mayra A., Elizabeth Carvajal-Millan, Jesús Ortega-García, Lubitza B. Santiago-Gómez, Yubia De Anda-Flores, Karla G. Martínez-Robinson, and Dora E. Valencia-Rivera. 2020. "Effect of Ultrasound-Treated Arabinoxylans on the Oxidative Stability of Soybean Oil" Antioxidants 9, no. 2: 147. https://doi.org/10.3390/antiox9020147