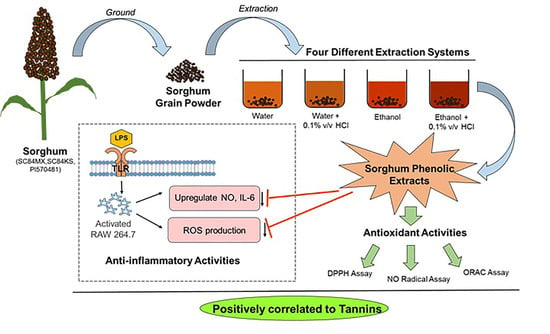
A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorghum Phenolics Extraction
2.3. Quantification of Phenolic Extracts
2.3.1. Measurement of Total Soluble Polyphenols
2.3.2. Measurement of Total Flavonoids
2.3.3. Measurement of Total Tannins
2.3.4. Measurement of Total Anthocyanins and 3-Deoxyanthocyanidins
2.4. HPLC Analysis of Phenolic Compounds
2.5. Measurement of Antioxidant Activity
2.5.1. NO Scavenging Assay
2.5.2. DPPH Radical Scavenging Assay
2.5.3. ORAC Assay
2.6. Anti-Inflammatory Activity in LPS-Induced RAW 264.7 Macrophages
2.6.1. Cell Culture and Cell Proliferation
2.6.2. Measurement of Pro-Inflammatory Markers
2.6.3. Measurement of Intracellular Reactive Oxygen Species
2.7. Statistical Analysis
3. Results
3.1. Sorghum Phenolic Contents in Different Extracting Systems
3.2. Sorghum Extracts Exert Antioxidant Activity
3.3. Sorghum Extracts Alleviate LPS-Induced Inflammation in RAW 264.7 Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Cardoso, L.D.M.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant′Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef] [Green Version]
- Rooney, L.W.; Waniska, R.D. Sorghum food and industrial utilization. Sorghum Orig. Hist. Technol. Prod. 2000, 689–729. [Google Scholar]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-deoxyanthocyanins from sorghum. J. Agric. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Ojwang, L.O.; Awika, J.M. Stability of apigeninidin and its methoxylated derivatives in the presence of sulfites. J. Agric. Food Chem. 2010, 58, 9077–9082. [Google Scholar] [CrossRef] [PubMed]
- Ojwang, L.; Awika, J.M. Effect of pyruvic acid and ascorbic acid on stability of 3-deoxyanthocyanidins. J. Sci. Food Agric. 2008, 88, 1987–1996. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Xiang, W.; Zhu, C.; Lin, Z.; Wu, Y.; Li, J.; Pandravada, S.; Ridder, D.D.; Bai, G. Presence of tannins in sorghum grains is conditioned by different natural alleles of Tannin1. Proc. Natl. Acad. Sci. USA 2012, 109, 10281–10286. [Google Scholar] [CrossRef] [Green Version]
- Dia, V.P.; Bradwell, J.; Pangloli, P. Sorghum Phenolics Inhibits Inflammasomes in Lipopolysaccharide (LPS)-Primed and Adenosine Triphosphate (ATP)-Activated Macrophages. Plant Foods Hum. Nutr. 2019, 74, 307–315. [Google Scholar] [CrossRef]
- Massey, A.R.; Reddivari, L.; Vanamala, J. The dermal layer of sweet sorghum (Sorghum bicolor) stalk, a byproduct of biofuel production and source of unique 3-deoxyanthocyanidins, has more antiproliferative and proapoptotic activity than the pith in p53 variants of HCT116 and colon cancer stem cells. J. Agric. Food Chem. 2014, 62, 3150–3159. [Google Scholar] [CrossRef]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agric. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef]
- Smolensky, D.; Rhodes, D.; McVey, D.S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-polyphenol sorghum bran extract inhibits cancer cell growth through ROS induction, cell cycle arrest, and apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Noronha, L.; Herald, T.; Bean, S.; Lee, S.-H.; Perumal, R.; Wang, W.; Smolensky, D. Evaluation of ethanol-based extraction conditions of sorghum bran bioactive compounds with downstream anti-proliferative properties in human cancer cells. Heliyon 2019, 5, e01589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Lee, J.; Herald, T.; Cox, S.; Noronha, L.; Perumal, R.; Lee, H.-S.; Smolensky, D. Anticancer Activity of a Novel High Phenolic Sorghum Bran in Human Colon Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A.K.; Smolensky, D.; Ngwaga, T.; Chauhan, D.; Cox, S.; Perumal, R.; Noronha, L.E.; Shames, S.R. High-polyphenol extracts from Sorghum bicolor attenuate replication of Legionella pneumophila within RAW 264.7 macrophages. FEMS Microbiol. Lett. 2020, 367, 053. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K.; Dowd, C. Inhibition of aromatase and α-amylase by flavonoids and proanthocyanidins from Sorghum bicolor bran extracts. J. Med. Food 2011, 14, 799–807. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Zuidema, M.Y.; Hannink, M.; Zhang, C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J. Cardiol. 2011, 3, 18–24. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Q.; Cao, J.; Xie, N.; Liu, K.; Shou, P.; Qian, F.; Wang, Y.; Shi, Y. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death. Dis. 2016, 7, e2167. [Google Scholar] [CrossRef] [Green Version]
- Lepedda, A.J.; Lobina, O.; Rocchiccioli, S.; Nieddu, G.; Ucciferri, N.; De Muro, P.; Idini, M.; Nguyen, H.Q.T.; Guarino, A.; Spirito, R. Identification of differentially expressed plasma proteins in atherosclerotic patients with type 2 diabetes. J. Diabetes Complicat. 2016, 30, 880–886. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. Rev. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Bartsch, H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1994, 305, 253–264. [Google Scholar] [CrossRef]
- Hibbs, J., Jr. Synthesis of nitric oxide from L-arginine: A recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res. Immunol. 1991, 142, 565–569. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharad, S.; Kapur, S. Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J. Indian. Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- Dia, V.P.; Wang, Z.; West, M.; Singh, V.; West, L.; De Mejia, E.G. Processing method and corn cultivar affected anthocyanin concentration from dried distillers grains with solubles. J. Agric. Food Chem. 2015, 63, 3205–3218. [Google Scholar] [CrossRef]
- Dia, V.P.; Pangloli, P.; Jones, L.; McClure, A.; Patel, A. Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 2016, 7, 3410–3420. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-throughput micro-plate HCl–vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef]
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Pangloli, P.; Dia, V.P. Impact of ultrasonication on the physicochemical properties of sorghum kafirin and in vitro pepsin-pancreatin digestibility of sorghum gluten-like flour. Food Chem. 2018, 240, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Pangloli, P.; Dia, V.P. Pepsin–pancreatin hydrolysis reduced the ability of lunasin-enriched material to inhibit activation of the inflammasomes in THP-1 human macrophages. Food Funct. 2017, 8, 4449–4458. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liang, Q.; Li, H.; Zhao, G. Solvent effects on phenolic content, composition, and antioxidant activity of extracts from florets of sunflower (Helianthus annuus L.). Ind. Crop. Prod. 2015, 76, 574–581. [Google Scholar] [CrossRef]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Tannin extract of guava leaves (Psidium guajava L) variation with concentration organic solvents. Intl. J. Sci. Tech. Res. 2013, 2, 106–110. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Shelembe, J.S.; Cromarty, D.; Bester, M.; Minnaar, A.; Duodu, K.G. Effect of acidic condition on phenolic composition and antioxidant potential of aqueous extracts from sorghum (Sorghum bicolor) bran. J. Food Biochem. 2014, 38, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 128, 173–179. [Google Scholar] [CrossRef]
- Haghi, G.; Hatami, A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J. Agric. Food Chem. 2010, 58, 10812–10816. [Google Scholar] [CrossRef] [PubMed]
- Bridgers, E.N.; Chinn, M.S.; Truong, V.-D. Extraction of anthocyanins from industrial purple-fleshed sweetpotatoes and enzymatic hydrolysis of residues for fermentable sugars. Ind. Crop. Prod. 2010, 32, 613–620. [Google Scholar] [CrossRef]
- Taleon, V.; Dykes, L.; Rooney, W.; Rooney, L. Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J. Cereal Sci. 2012, 56, 470–475. [Google Scholar] [CrossRef]
- Moraes, É.A.; da Silva Marineli, R.; Lenquiste, S.A.; Steel, C.J.; de Menezes, C.B.; Queiroz, V.A.V.; Júnior, M.R.M. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Sohounhloue, D.C. Optimization of the Extraction of Sorghum’s Polyphenols for Industrial Production by Membrane Processes. Res. J. Recent Sci. 2012, 1, 2502. [Google Scholar]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef]
- Moraes, É.A.; Natal, D.I.G.; Queiroz, V.A.V.; Schaffert, R.E.; Cecon, P.R.; de Paula, S.O.; dos Anjos Benjamim, L.; Ribeiro, S.M.R.; Martino, H.S.D. Sorghum genotype may reduce low-grade inflammatory response and oxidative stress and maintains jejunum morphology of rats fed a hyperlipidic diet. Food Res. Int. 2012, 49, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Awika, J.M.; Yang, L.; Browning, J.D.; Faraj, A. Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT Food Sci. Technol. 2009, 42, 1041–1046. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, W.L.; Rooney, L.W. Evaluation of phenolics and antioxidant activity of black sorghum hybrids. J. Cereal Sci. 2013, 58, 278–283. [Google Scholar] [CrossRef]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.-H.; Zhao, B.T.; Lee, J.H.; Kim, Y.H.; Min, B.S.; Woo, M.H. Isolation of benzoic and cinnamic acid derivatives from the grains of Sorghum bicolor and their inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells. Food Chem. 2015, 168, 512–519. [Google Scholar] [CrossRef]
- Burdette, A.; Garner, P.L.; Mayer, E.P.; Hargrove, J.L.; Hartle, D.K.; Greenspan, P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J. Med. Food 2010, 13, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of hyaluronidase activity by select sorghum brans. J. Med. Food 2008, 11, 307–312. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [Green Version]




| Sorghum Sample | Extract without HCl | Extract with 0.1% v/v HCl | ||
|---|---|---|---|---|
| Water Extraction | Ethanol Extraction | Water Extraction | Ethanol Extraction | |
| Total Polyphenols, mg gallic acid eq/g | ||||
| SC84MX | 8.55 ± 0.07 f | 9.58 ± 0.52 de | 9.00 ± 0.2 ef | 18.26 ± 0.79 b |
| SC84KS | 8.23 ± 0.44 f | 10.24 ± 0.25 d | 8.50 ± 0.11 f | 19.60 ± 0.88 a |
| PI570481 | 1.42 ± 0.05 i | 6.02 ± 0.33 g | 3.24 ± 0.13 h | 12.61 ± 1.07 c |
| Total Flavonoids, mg quercetin eq/g | ||||
| SC84MX | 1.13 ± 0.02 cd | 1.29 ± 0.32 bc | 0.79 ± 0.06 e | 1.74 ± 0.01 a |
| SC84KS | 0.89 ± 0.04 de | 1.18 ± 0.20 cd | 0.72 ± 0.02 e | 1.65 ± 0.03 a |
| PI570481 | 0.57 ± 0.08 e | 0.64 ± 0.08 e | 0.59 ± 0.08 e | 1.55 ± 0.01 ab |
| Total Tannins, mg epicatechin/g | ||||
| SC84MX | 21.96 ± 0.64 d | 131.11 ± 8.39 a | 5.28 ± 0.24 e | 66.06 ± 3.78 c |
| SC84KS | 21.22 ± 1.11 d | 136.11 ± 12.06 a | 6.17 ± 0.08 e | 58.82 ± 0.37 c |
| PI570481 | 11.11 ± 1.92 e | 99.44 ± 10.72 b | 6.03 ± 1.16 e | 62.80 ± 3.59 c |
| Total 3-deoxyanthocyanidins, μg luteolinidin eq/g | ||||
| SC84MX | 127.40 ± 2.04 b | 29.72 ± 0.92 g | 28.40 ± 3.25 g | 92.54 ± 1.07 d |
| SC84KS | 97.93 ± 1.08 d | 35.82 ± 0.40 f | 35.64 ± 6.51 f | 112.63 ± 1.55 c |
| PI570481 | 18.31 ± 0.89 h | 68.37 ± 0.15 e | 16.86 ± 1.82 h | 325.23 ± 4.76 a |
| Total Anthocyanins, μg cyanidin-3-glucoside eq/g | ||||
| SC84MX | 168.05 ± 1.66 b | 48.14 ± 0.77 g | 34.55 ± 3.60 h | 104.66 ± 1.46 e |
| SC84KS | 126.88 ± 1.41 d | 64.49 ± 1.45 f | 48.49 ± 8.15 g | 126.27 ± 2.77 d |
| PI570481 | 38.86 ± 1.61 h | 149.24 ± 0.45 c | 26.80 ± 0.73 i | 554.87 ± 7.38 a |
| Standards | WL (nm) | RT (min) | Sample Area | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Extracts | Ethanol Extracts | Water Extracts | Ethanol Extracts | Water Extracts | Ethanol Extracts | |||||||||
| MX | MX-HCl | MX | MX-HCl | KS | KS-HCl | KS | KS-HCl | PI | PI-HCl | PI | PI-HCl | |||
| Gallic acid | 280 | 1.81 | 1346.4 | 1690.5 | 226.7 | 964.9 | 1527.7 | 1737.3 | 321.7 | 825.2 | 1517.4 | 1413.8 | 282.4 | 261.3 |
| Catechin | 280 | 3.17 | 2963.5 | 2143.5 | 5557.2 | N/D | 7842.0 | N/D | 6032.3 | N/D | N/D | N/D | N/D | N/D |
| Vanillic acid | 280 | 4.77 | 2816.0 | 1935.0 | 2157.0 | 726.0 | 4154.4 | 1451.3 | 3181.8 | 861.2 | 718.4 | 404.7 | 3846.2 | N/D |
| Syringic acid | 280 | 4.90 | 1101.0 | 986.0 | N/D | N/D | N/D | 540.9 | N/D | N/D | N/D | 131.6 | N/D | N/D |
| Coumaric acid | 280 | 7.03 | 2507.0 | N/D | 3658.4 | 248.2 | 2701.9 | 1923.6 | 3994.5 | 322.8 | 497.4 | 179.2 | 1631.5 | N/D |
| Ellagic acid | 280 | 9.46 | 8201.7 | 8110.6 | 3200.5 | 1616.5 | 8974.0 | 3549.6 | 4303.9 | 2148.3 | N/D | N/D | 386.6 | 128.1 |
| Eriodictyol | 280 | 21.75 | 139.5 | 290.3 | N/D | 423.0 | 116.4 | N/D | N/D | 169.0 | N/D | N/D | N/D | N/D |
| Naringenin | 280 | 28.08 | 1108.6 | 264.0 | N/D | 284.3 | 929.9 | N/D | N/D | 271.0 | N/D | N/D | N/D | N/D |
| Chrysin | 280 | 37.45 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Caffeic acid | 340 | 4.47 | 594.7 | 483.8 | 262.2 | 138.2 | 565.8 | 507.3 | 437.2 | 242.4 | 396.0 | 159.2 | 324.5 | N/D |
| Ferulic acid | 340 | 9.35 | 1239.1 | 1081.2 | 301.9 | 365.7 | 1448.4 | 506.9 | 468.6 | 458.8 | N/D | N/D | N/D | N/D |
| Sinapic acid | 340 | 9.89 | 302.6 | N/D | 195.9 | N/D | N/D | 177.4 | 182.7 | N/D | 358.2 | 115.6 | N/D | N/D |
| Quercetin | 340 | 24.88 | N/D | 56.9 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Luteolin | 340 | 25.45 | N/D | N/D | 106.0 | N/D | N/D | N/D | 128.5 | N/D | N/D | N/D | 91.2 | N/D |
| Apigenin | 340 | 29.65 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Parameter | Parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TF | TT | T3DA | TA | NO | DPPH | ORAC | RAW-NO | IL-6 | ROS | |
| TP | 1 | 0.911 ** (<0.0001) | 0.313 (0.323) | 0.420 (0.174) | 0.289 (0.363) | −0.496 (0.101) | 0.227 (0.478) | −0.123 (0.703) | 0.430 (0.163) | 0.128 (0.692) | 0.337 (0.284) |
| TF | 1 | 0.443 (0.149) | 0.563 (0.057) | 0.452 (0.140) | −0.315 (0.318) | 0.339 (0.281) | −0.248 (0.437) | 0.514 (0.087) | 0.257 (0.420) | 0.415 (0.180) | |
| TT | 1 | 0.059 (0.856) | 0.114 (0.724) | −0.341 (0.279) | 0.631 * (0.028) | −0.379 (0.224) | 0.730 ** (0.007) | 0.649 * (0.022) | 0.890 ** (<0.0001) | ||
| T3DA | 1 | 0.980 ** (<0.0001) | 0.149 (0.643) | 0.253 (0.428) | −0.774 ** (0.003) | 0.313 (0.322) | −0.005 (0.987) | 0.221 (0.491) | |||
| TA | 1 | 0.188 (0.558) | 0.353 (0.260) | −0.855 ** (<0.0001) | 0.378 (0.225) | 0.080 (0.806) | 0.298 (0.348) | ||||
| NO | 1 | −0.163 (0.613) | −0.126 (0.693) | −0.289 (0.362) | −0.066 (0.839) | −0.342 (0.277) | |||||
| DPPH | 1 | −0.518 (0.085) | 0.937 ** (<0.0001) | 0.896 ** (<0.0001) | 0.724 ** (0.008) | ||||||
| ORAC | 1 | −0.541 (0.070) | −0.301 (0.342) | −0.539 (0.071) | |||||||
| RAW-NO | 1 | 0.862 ** (<0.0001) | 0.750 ** (0.005) | ||||||||
| IL-6 | 1 | 0.630 * (0.028) | |||||||||
| ROS | 1 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants 2020, 9, 1297. https://doi.org/10.3390/antiox9121297
Hong S, Pangloli P, Perumal R, Cox S, Noronha LE, Dia VP, Smolensky D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants. 2020; 9(12):1297. https://doi.org/10.3390/antiox9121297
Chicago/Turabian StyleHong, Shan, Philipus Pangloli, Ramasamy Perumal, Sarah Cox, Leela E. Noronha, Vermont P Dia, and Dmitriy Smolensky. 2020. "A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages" Antioxidants 9, no. 12: 1297. https://doi.org/10.3390/antiox9121297