N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature
Abstract
:1. Introduction
2. Methods for Study Inclusion
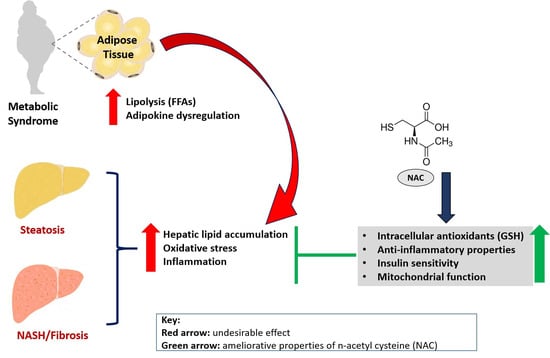
3. A Brief Overview on NAFLD and Implicated Pathophysiological Mechanisms
4. An Overview of Evidence on the Impact of NAC on NAFLD-Related Complications
4.1. NAC Targets Hepatic Lipid Accumulation to Improve Liver Function in Preclinical Models of NAFLD
| Author, Year | Experimental Model, NAC Dosage, and Intervention Period | Main Findings |
|---|---|---|
| Lin et al., 2004 [40] | Male Balb/cA mice consuming a high saturated fat diet (with 18% saturated fat) received NAC (1 g/L) in drinking water for 4 weeks. | NAC significantly reduced malic enzyme and fatty acid synthase activities, and significantly lowered TG levels in the plasma and liver. NAC also reduced cholesterol levels in the plasma and liver and improved high saturated fat diet-related hyperglycaemia, hyperuricemia, and oxidation stress. |
| Samuhasaneeto et al., 2007 [41] | Male Sprague-Dawley rats fed high fat diet (HFD) for 6 weeks then given 500 mg/kg/day of NAC for 4 weeks. | Treatment with diet or diet plus NAC reduced the levels of cholesterol back to normal. Liver sections from NAC treatment showed a decrease in fat. |
| Lin and Yin, 2008 [44] | NAFLD was induced through HFD in male C57BL/6 mice, whilst NAC (1 g/L) was directly added into the drinking water as a supplement for 4 weeks. | NAC significantly decreased triacylglycerides and total cholesterol levels via lowering the activity and mRNA expression of lipogenic-related enzymes. NAC also suppressed high saturated fat-induced hepatic mRNA expression of sterol regulatory element-binding protein (SREBP)-1c and SREBP-2. |
| Korou et al., 2010 [47] | Male C57bl/6 mice received the test diet with NAC supplementation (230 mg/kg body weight) and the high cholesterol group was fed the test diet enriched with 10% sesame oil for 8 weeks. | NAC reduced lipid levels. In terms of liver histology, the lesions observed in the NAC-treated animals were less severe than those seen in the other high cholesterol groups. |
| Lai et al., 2012 [45] | Male Sprague-Dawley rats fed a modified diet supplemented with 1.0% NAC. After one week, rats on each diet were exposed to 0, 1, or 5 μmol/kg body weight PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl)) by i.p. injection and euthanized 2 weeks later. | NAC resulted in a reduction in hepatocellular lipid in both PCB groups. This effect was confirmed by the gravimetric analysis of extracted lipids. The expression of CD36, a scavenger receptor involved in regulating hepatic fatty acid uptake, was reduced with high-dose PCB treatment but unaltered in PCB-treated rats on an NAC-supplemented diet. |
| El-Lakkany et al., 2016 [42] | NAFLD was induced by HFD for 12 weeks in male Sprague-Dawley rats before treatment with metformin at a dose of 150 mg/kg, NAC at a dose of 500 mg/kg or metformin for 8 weeks. | NAC or metformin individually improved most of these biochemical and histological parameters related to hepatic steatosis such as lobular inflammation, fibrosis accompanied with elevated serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase, cholesterol, triglycerides, LDL, VLDL, leptin, TNF-α, and TGF-β1. These improvements were more pronounced in the combination treatment. |
| Ma et al., 2016 [48] | Six-week-old male C57BL/6 mice fed on chow or HFD were treated with NAC (2 g/L) in drinking water for 11 weeks. | NAC blocked fat mass and the development of obesity reducing HFD-induced macrophage infiltration, and enhanced adiponectin gene expression. NAC oral administration suppressed hepatic lipid accumulation, as evidenced by lower levels of triglyceride and cholesterol in the liver. The beneficial effects are associated with a decrease in hepatic peroxisome proliferator-activated receptor (PPAR)γ and its target gene expression. |
| Machado et al., 2016 [43] | NASH was induced by methionine–choline deficient diet for 8 weeks. NAC was administered in the drinking water, resulting in an estimated consumption of 250 mg/kg body 3 times a week. | NAC failed to inhibit caspase-2 activation, or improve NASH, normalize pantothenate kinase expression, or restore free CoA levels. |
| Zhou et al., 2017 [49] | Male Sprague-Dawley fed HFD and received NAC (60 mg/kg) or NAC-activated carbon sustained-release microcapsule (ACNAC; 15, 30 and 60 mg/kg) by gastric perfusion daily for 7 weeks. | ACNAC exhibited different degrees of improvement in various indexes such as reducing the activity of ALT, AST and the content of total cholesterol (TC), TG, LDL-C, increased the content of HDL-C and strengthened dipeptidyl peptidase IV protein expression in the liver cell membrane. |
| Stojanović et al., 2018 [46] | Wistar rats received methionine (0.8 mmol/kg/day) + NAC (50 mg/kg/day i.p) for 21 days. | Methionine reduced AST, ALT, and ALP activity, whilst the NAC application increased activity of antioxidative enzymes and prevented intensive histological changes in the liver. |
| Shen et al., 2019 [50] | Male Sprague-Dawley rats received NAC (2.4 mmol/kg) for 3 days before D-galactosamine (400 mg/kg). | NAC reduced liver cholesterol, with fish oil showing a greater attenuating effect than NAC. |
4.2. NAC Targets Oxidative Stress and Inflammation to Improve Liver Function in Preclinical Models of NAFLD
| Author, Year | Experimental Model, NAC Dosage, and Intervention Period | Main Findings |
|---|---|---|
| Nakano et al., 1997; 1998 [53,54] | NAC was administered in a ready-to-use solution, at 150 mg/kg body weight in Wistar rats fed an MCD diet, through the mesenteric vein 15 min before liver harvest. | Addition of NAC to the liver before cold storage significantly improved glutathione (GSH) levels and ameliorated steatotic livers. This effect was associated in part by the reversal of hypothermic ischemic-reperfusion injury and the amelioration of oxidative stress. |
| Fusai et al., 2005 [60] | New Zealand White rabbits were fed a high-cholesterol (2%) diet. After, an intravenous infusion of NAC (150 mg/kg of body weight) was administered prior to and during the 6 h reperfusion period. | NAC administration significantly improved portal flow, hepatic microcirculation, bile composition and bile flow after 5 h of reperfusion. NAC administration was also associated with less hepatocellular injury, as indicated by ALT serum activity, and decreased the oxidation of dihydrorhodamine to rhodamine. |
| de Oliveira et al., 2006 [55] | NAFLD was induced in Wistar male rats by a choline-deficient diet for 4 weeks before the oral administration of S-nitroso-N-acetylcysteine (SNAC; 1.4 mg/kg/day in comparison to those on PBS solution, NAC solution (7 mg/kg/d) for 4 weeks. | The absence of NAFLD in the SNAC-treated group was positively correlated with a decrease in the concentration of forming reactive fatty acid hydroperoxides in the liver homogenate, compared to the control group, while serum levels of aminotransferases were unaltered. |
| Samuhasaneeto et al., 2007 [41] | Male Sprague-Dawley rats were fed high fat diet (HFD) for 6 weeks then switched to regular dry rat chow + 20 or 500 mg/kg/day of NAC for 4 weeks. | Treatment with a diet or diet plus NAC reduced the levels of GSH, cholesterol, and hepatic MDA back to normal. Liver sections from groups 3–5 showed a decrease in fat deposition and necro-inflammation in hepatocytes. |
| Thong-Ngam et al., 2007 [61] | Male Sprague-Dawley rats were fed HFD plus 20 mg/kg per day of NAC orally for 6 weeks. | NAC treatment improved the level of GSH but did not affect MDA. NAC also led to a decrease in fat deposition and necro-inflammation. |
| Baumgardner et al., 2008 [51] | NAFLD induced by overfeeding Sprague-Dawley rats with dietary polyunsaturated fat containing 70% corn oil with or without 2 g/kg NAC (intragastric gavage) for 65 days. | NAC prevented many aspects of NAFLD progression by decreasing the development of oxidative stress and subsequent increases in TNF-α but did not block the development of steatosis. |
| Uzun et al., 2009 [59] | NAFLD in male Wistar rats was induced with a choline-deficient diet for 4 weeks. NAC (100 mg/mL) was administered intraperitoneally at a dosage of 100 mg/kg per day twice a day, at 08:00 and 20:00 h. | NAC enhanced regeneration after partial hepatectomy in rats with NAFLD by enhancing GSH content while reducing MDA levels. |
| Korou et al., 2010 [47] | Male C57bl/6 mice received the test diet with NAC supplementation (230 mg/kg body weight) and high cholesterol group fed the test diet enriched with 10% sesame oil for 8 weeks. | NAC reduced lipid levels, concomitant to decreasing serum lipid peroxidation and restored nitric oxide bioavailability. In terms of liver histology, the lesions observed in the NAC-treated animals were less severe than those seen in the other high cholesterol groups. |
| Mazo et al., 2013 [17] | Non-alcoholic steatohepatitis (NASH)-induced in Sprague-Dawley rats fed with a choline-deficient, high trans-fat diet and exposed to diethylnitrosamine for 8 weeks. Animals received SNAC daily by gavage (8.0 μmol/kg = 1.4 mg/kg) during an 8 week period. | SNAC led to a 34.4% reduction in the collagen-occupied area associated with upregulation of matrix metalloproteinases (MMP)-13 and -9 and the downregulation of heat-shock protein (HSP)-60, tissue inhibitors of metalloproteinase-2, transforming growth factor (TGF)β-1, and collagen-1α. |
| Ali et al., 2016 [56] | NAFLD was induced by feeding male Wistar rats a MCD diet for four cycles before treatment with NAC (20 mg/kg/d), ursodeoxycholic acid + resveratrol, and ursodeoxycholic acid + NAC orally for 28 days. | Resveratrol and NAC administration significantly improved liver index (resveratrol only), alanine transaminase, TNF-α, glucose, albumin, malondialdehyde (MDA), GSH, glutathione-S-transferase, total cholesterol, low-density lipoprotein-c, and leptin levels compared with steatosis control values. |
| Ma et al., 2016 [48] | Six-week-old male C57BL/6 mice fed a chow or HFD were treated with NAC (2 g/L) in drinking water for 11 weeks. | NAC blocked the fat mass and HFD-induced macrophage infiltration, and enhanced adiponectin gene expression. NAC suppressed hepatic lipid accumulation, as evidenced by lower levels of triglyceride and cholesterol in the liver. The beneficial effects are associated with a decrease in hepatic peroxisome proliferator-activated receptor (PPAR)γ and its target gene expression. |
| de Oliveira Rosa et al., 2018 [62] | Streptozotocin-induced diabetic Wistar rats received NAC at 25 mg/kg body weight daily, orally via gavage, for 37 days. | NAC improved hyperglycaemia and hypoinsulinemia, as well as reducing serum ALT and urea, hepatic triglycerides accumulation and oxidative stress biomarkers in the diabetic liver, as well as improving hepatic antioxidant enzymes’ activities, especially restoring GSH content. |
| Shi et al., 2018 [58] | Three-week old male Sprague Dawley rats were given (intragastrically) high-fat diet (HFD) with/without activated carbon-NAC (ACNAC) treatment (20, 40 and 80 mg/kg) for 7 consecutive weeks. | ACNAC supplementation improved liver pathologies and prevented HFD-induced telomere shortening and improved telomerase activity. ACNAC supplementation also increased the expression of B-cell lymphoma 2 (Bcl-2), but reduced that of Bax and caspase-3. |
| Wang et al., 2018 [16] | Male Sprague-Dawley rats were fed with HFD to produce the NASH model and treated (intraperitoneal injections) with or without N,N’-diacetylcystine (DiNAC) at 12.5 mg/kg, 25 mg/kg, and 50 mg/kg body for 8 weeks. | DiNAC reduced the levels of ALT and AST. DiNAC alleviated histological injury. Moreover, DiNAC strongly reduced the generation of inflammatory cytokines, such as interleukin-6 (IL-6), TNF-α and interleukin-1β (IL-1β), through nuclear factor kappa B (NF-κB) downregulation. |
| Tsai et al., 2020 [52] | NAFLD was induced in male C57BL/6 (B6) mice by administering HF diet for 12 months with NAC (10 mM NAC) dissolved in water for 6 or 12 months. | NAC intake for 6 or 12 months decreased liver steatosis. NAC treatment also reduced cellular apoptosis and caspase-3 expression. With regards to endoplasmic reticulum stress, only treatment at 12 months improved/reduced phospho-protein kinase R-like endoplasmic reticulum kinase and activating transcription factor 4 expression. |
4.3. Impact of NAC on Lipid Accumulation, Oxidative Stress and Inflammation in Knockout Models of NAFLD
4.4. Clinical Evidence on the Impact of NAC on NAFLD-Associated Complications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016, 64, 1082–1086. [Google Scholar] [CrossRef]
- McMorrow, A.M.; Connaughton, R.M.; Lithander, F.E.; Roche, H.M. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: Potential impact of dietary fat quality. Proc. Nutr. Soc. 2015, 74, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar] [PubMed]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Sherif, Z.A.; Saeed, A.; Ghavimi, S.; Nouraie, S.M.; Laiyemo, A.O.; Brim, H.; Ashktorab, H. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig. Dis. Sci. 2016, 61, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Gentric, G.; Maillet, V.; Paradis, V.; Couton, D.; L’Hermitte, A.; Panasyuk, G.; Fromenty, B.; Celton-Morizur, S.; Desdouets, C. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.; Uddin, M.J.; Pak, E.S.; Kang, H.; Jin, E.J.; Jo, S.; Kang, D.; Lee, H.; Ha, H. The impaired redox balance in peroxisomes of catalase knockout mice accelerates nonalcoholic fatty liver disease through endoplasmic reticulum stress. Free Radic. Biol. Med. 2020, 148, 22–32. [Google Scholar] [CrossRef]
- Ore, A.; Akinloye, O.A. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina 2019, 55, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Tacke, F. Interleukins in chronic liver disease: Lessons learned from experimental mouse models. Clin. Exp. Gastroenterol. 2014, 7, 297–306. [Google Scholar] [PubMed] [Green Version]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The differential roles of T cells in non-alcoholic fatty liver disease and obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.B.; Chen, W.; Huang, F.F.; Zhang, J.F. Elevated Th22 cells correlated with Th17 cells in patients with high liver stiffness in nonalcoholic fatty liver disease. Eur. J. Inflamm. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Zhuang, R.; Bao, J.; Shen, Y.; Xi, J.; Sun, J.; Fang, H. N,N′-diacetylcystine ameliorates inflammation in experimental non-alcoholic steatohepatitis by regulating nuclear transcription factor kappa B activation. Int. J. Clin. Exp. Pathol. 2018, 11, 5351–5358. [Google Scholar]
- Mazo, D.F.; de Oliveira, M.G.; Pereira, I.V.; Cogliati, B.; Stefano, J.T.; de Souza, G.F.; Rabelo, F.; Lima, F.R.; Ferreira Alves, V.A.; Carrilho, F.J.; et al. S-nitroso-N-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des. Devel. Ther. 2013, 7, 553–563. [Google Scholar]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [Green Version]
- Bastin, A.J.; Davies, N.; Lim, E.; Quinlan, G.J.; Griffiths, M.J. Systemic inflammation and oxidative stress post-lung resection: Effect of pretreatment with N-acetylcysteine. Respirology 2016, 21, 180–187. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, S.C.; Zaretsky, M.D.; Dubs, J.G.; Roederer, M.; Anderson, M.; Green, A.; Mitra, D.; Watanabe, N.; Nakamura, H.; Tjioe, I.; et al. N-acetylcysteine replenishes glutathione in HIV infection. Eur. J. Clin. Investig. 2000, 30, 915–929. [Google Scholar] [CrossRef]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic review on the protective effect of n-acetyl cysteine against diabetes-associated cardiovascular complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Orlando, P.; Silvestri, S.; Mazibuko-Mbeje, S.E.; Johnson, R.; Marcheggiani, F.; Cirilli, I.; Muller, C.J.F.; Louw, J.; Obonye, N.; et al. N-Acetyl cysteine ameliorates hyperglycemia-induced cardiomyocyte toxicity by improving mitochondrial energetics and enhancing endogenous Coenzyme Q9/10 levels. Toxicol. Rep. 2019, 6, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N.; Dear, J.W. Acetylcysteine in paracetamol poisoning: A perspective of 45 years of use. Toxicol. Res. 2019, 8, 489–498. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araújo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of n-acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Dias, S.C.; Johnson, R. Cardioprotective potential of N-acetyl cysteine against hyperglycaemia-induced oxidative damage: A protocol for a systematic review. Syst. Rev. 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and prevention of hepatic steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Vuppalanchi, R.; Chalasani, N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009, 49, 306–317. [Google Scholar] [CrossRef]
- Arab, J.P.; Arrese, M.; Shah, V.H. Gut microbiota in non-alcoholic fatty liver disease and alcohol-related liver disease: Current concepts and perspectives. Hepatol. Res. 2020, 50, 407–418. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, C.A.; Klett, E.L.; Coleman, R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009, 50 (Suppl.), S74–S79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.H. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [Green Version]
- García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.P.; Liu, X.J.; Xie, L.; Shen, X.Z.; Wu, J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab. Investig. 2019, 99, 749–763. [Google Scholar] [CrossRef]
- Lin, C.C.; Yin, M.C.; Hsu, C.C.; Lin, M.P. Effect of five cysteine-containing compounds on three lipogenic enzymes in Balb/cA mice consuming a high saturated fat diet. Lipids 2004, 39, 843–848. [Google Scholar] [CrossRef]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Patumraj, S.; Klaikeaw, N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis. J. Med. Assoc. Thai. 2007, 90, 788–797. [Google Scholar] [PubMed]
- El-Lakkany, N.M.; Seif El-Din, S.H.; Sabra, A.A.; Hammam, O.A.; Ebeid, F.A. Co-administration of metformin and N-acetylcysteine with dietary control improves the biochemical and histological manifestations in rats with non-alcoholic fatty liver. Res. Pharm. Sci. 2016, 11, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.V.; Kruger, L.; Jewell, M.L.; Michelotti, G.A.; Pereira Tde, A.; Xie, G.; Moylan, C.A.; Diehl, A.M. Vitamin B5 and n-acetylcysteine in nonalcoholic steatohepatitis: A preclinical study in a dietary mouse model. Dig. Dis. Sci. 2016, 61, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Yin, M.C. Effects of cysteine-containing compounds on biosynthesis of triacylglycerol and cholesterol and anti-oxidative protection in liver from mice consuming a high-fat diet. Br. J. Nutr. 2008, 99, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, I.K.; Dhakal, K.; Gadupudi, G.S.; Li, M.; Ludewig, G.; Robertson, L.W.; Olivier, A.K. N-acetylcysteine (NAC) diminishes the severity of PCB 126-induced fatty liver in male rodents. Toxicology 2012, 302, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanović, M.; Todorović, D.; Šćepanović, L.; Mitrović, D.; Borozan, S.; Dragutinović, V.; Labudović-Borović, M.; Krstić, D.; Čolović, M.; Djuric, D. Subchronic methionine load induces oxidative stress and provokes biochemical and histological changes in the rat liver tissue. Mol. Cell. Biochem. 2018, 448, 43–50. [Google Scholar] [CrossRef]
- Korou, L.M.; Agrogiannis, G.; Pantopoulou, A.; Vlachos, I.S.; Iliopoulos, D.; Karatzas, T.; Perrea, D.N. Comparative antilipidemic effect of N-acetylcysteine and sesame oil administration in diet-induced hypercholesterolemic mice. Lipids Health Dis. 2010, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Gao, M.; Liu, D. N-acetylcysteine protects mice from high fat diet-induced metabolic disorders. Pharm. Res. 2016, 33, 2033–2042. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Shi, T.; Yan, J.; Chen, X.; Liao, L.; Zhao, S.; Fang, H.; Zhuang, R. Effects of activated carbon N-acetylcysteine sustained-release microcapsule on dipeptidyl peptidase IV expression in young rats with non-alcoholic fatty liver disease. Exp. Ther. Med. 2017, 14, 4737–4744. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Lau-Cam, C.A. Taurine enhances the protective actions of fish oil against D-galactosamine-induced metabolic changes and hepatic lipid accumulation and injury in the rat. In Taurine 11, Advances in Experimental Medicine and Biology; Springer Nature Pte Ltd.: Singapore, 2019; Volume 1155, pp. 71–85. [Google Scholar]
- Baumgardner, J.N.; Shankar, K.; Hennings, L.; Albano, E.; Badger, T.M.; Ronis, M.J. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J. Nutr. 2008, 138, 1872–1879. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Chen, Y.J.; Yu, H.R.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Sheen, J.M.; Wang, P.W.; Tiao, M.M. Long term N-acetylcysteine administration rescues liver steatosis via endoplasmic reticulum stress with unfolded protein response in mice. Lipids Health Dis. 2020, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nagasaki, H.; Barama, A.; Boudjema, K.; Jaeck, D.; Kumada, K.; Tatsuno, M.; Baek, Y.; Kitamura, N.; Suzuki, T.; et al. The effects of N-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the rat steatotic liver produced by a choline-methionine-deficient diet. Hepatology 1997, 26, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nagasaki, H.; Yoshida, K.; Kigawa, G.; Fujiwara, Y.; Kitamura, N.; Kuzume, M.; Takeuchi, S.; Sasaki, J.; Shimura, H.; et al. N-acetylcysteine and anti-ICAM-1 monoclonal antibody reduce ischemia-reperfusion injury of the steatotic rat liver. Transplant. Proc. 1998, 30, 3763. [Google Scholar] [CrossRef]
- de Oliveira, C.P.; Simplicio, F.I.; de Lima, V.M.; Yuahasi, K.; Lopasso, F.P.; Alves, V.A.; Abdalla, D.S.; Carrilho, F.J.; Laurindo, F.R.; de Oliveira, M.G. Oral administration of S-nitroso-N-acetylcysteine prevents the onset of non alcoholic fatty liver disease in rats. World J. Gastroenterol. 2006, 12, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Messiha, B.A.; Abdel-Latif, H.A. Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats. Pharm. Biol. 2016, 54, 1198–1208. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soejima, Y.; Fukusato, T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2300–2308. [Google Scholar] [CrossRef]
- Shi, T.; Yang, X.; Zhou, H.; Xi, J.; Sun, J.; Ke, Y.; Zhang, J.; Shao, Y.; Jiang, X.; Pan, X.; et al. Activated carbon N-acetylcysteine microcapsule protects against nonalcoholic fatty liver disease in young rats via activating telomerase and inhibiting apoptosis. PLoS ONE 2018, 13, e0189856. [Google Scholar] [CrossRef] [Green Version]
- Uzun, M.A.; Koksal, N.; Kadioglu, H.; Gunerhan, Y.; Aktas, S.; Dursun, N.; Sehirli, A.O. Effects of N-acetylcysteine on regeneration following partial hepatectomy in rats with nonalcoholic fatty liver disease. Surg. Today 2009, 39, 592–597. [Google Scholar] [CrossRef]
- Fusai, G.; Glantzounis, G.K.; Hafez, T.; Yang, W.; Quaglia, A.; Sheth, H.; Kanoria, S.; Parkes, H.; Seifalian, A.; Davidson, B.R. N-Acetylcysteine ameliorates the late phase of liver ischaemia/reperfusion injury in the rabbit with hepatic steatosis. Clin. Sci. 2005, 109, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Thong-Ngam, D.; Samuhasaneeto, S.; Kulaputana, O.; Klaikeaw, N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J. Gastroenterol. 2007, 13, 5127–5132. [Google Scholar] [CrossRef] [Green Version]
- Rosa, L.R.O.; Kaga, A.K.; Barbanera, P.O.; Queiroz, P.M.; do Carmo, N.O.L.; Fernandes, A.A.H. Beneficial effects of N-acetylcysteine on hepatic oxidative stress in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2018, 96, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, W.; Xu, Y.; Xu, J.; Zhao, D.; Ye, L.; Yu, G.; Wang, Z.; Lu, Q.; Lin, J.; Yang, T.; et al. Berberine inhibits Nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstee, Q.M.; Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006, 87, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Nicco, C.; Van Nhieu, J.T.; Borderie, D.; Chereau, C.; Conti, F.; Jaffray, P.; Soubrane, O.; Calmus, Y.; Weill, B.; et al. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology 2004, 39, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.P.; de Lima, V.M.; Simplicio, F.I.; Soriano, F.G.; de Mello, E.S.; de Souza, H.P.; Alves, V.A.; Laurindo, F.R.; Carrilho, F.J.; de Oliveira, M.G. Prevention and reversion of nonalcoholic steatohepatitis in OB/OB mice by S-nitroso-N-acetylcysteine treatment. J. Am. Coll. Nutr. 2008, 27, 299–305. [Google Scholar] [CrossRef]
- De Oliveira, C.P.; Stefano, J.T.; de Lima, V.M.; de Sa, S.V.; Simplicio, F.I.; de Mello, E.S.; Correa-Giannella, M.L.; Alves, V.A.; Laurindo, F.R.; de Oliveira, M.G.; et al. Hepatic gene expression profile associated with non-alcoholic steatohepatitis protection by S-nitroso-N-acetylcysteine in ob/ob mice. J. Hepatol. 2006, 45, 725–733. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Alves, V.A.; Lima, V.M.; Stefano, J.T.; Debbas, V.; Sa, S.V.; Wakamatsu, A.; Correa-Giannella, M.L.; de Mello, E.S.; Havaki, S.; et al. Modulation of hepatic microsomal triglyceride transfer protein (MTP) induced by S-nitroso-N-acetylcysteine in ob/ob mice. Biochem. Pharmacol. 2007, 74, 290–297. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Karns, R.; Gandhi, C.R. Augmenter of liver regeneration protein deficiency promotes hepatic steatosis by inducing oxidative stress and microRNA-540 expression. FASEB J. 2019, 33, 3825–3840. [Google Scholar] [CrossRef]
- Chen, Y.W.; Harris, R.A.; Hatahet, Z.; Chou, K.M. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2015, 112, E4556–E4564. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Homma, T.; Kurahashi, T.; Kang, E.S.; Fujii, J. Oxidative stress triggers lipid droplet accumulation in primary cultured hepatocytes by activating fatty acid synthesis. Biochem. Biophys. Res. Commun. 2015, 464, 229–235. [Google Scholar] [CrossRef]
- Preziosi, M.E.; Singh, S.; Valore, E.V.; Jung, G.; Popovic, B.; Poddar, M.; Nagarajan, S.; Ganz, T.; Monga, S.P. Mice lacking liver-specific β-catenin develop steatohepatitis and fibrosis after iron overload. J. Hepatol. 2017, 67, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflug. Arch. 2019, 471, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Sonsuz, A. N-acetylcsyteine in the treatment of non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2003, 18, 1214–1224. [Google Scholar]
- Mager, D.R.; Marcon, M.; Wales, P.; Pencharz, P.B. Use of N-acetyl cysteine for the treatment of parenteral nutrition-induced liver disease in children receiving home parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.P.; Cotrim, H.P.; Stefano, J.T.; Siqueira, A.C.G.; Salgado, A.L.A.; Parise, E.R. N-acetyl cysteine and/or usrsodeoxycholic acid associated with metformin in non-alcoholic steaohepatitis: An open label-label multicenter randomized controlled trial. Arq. Gastroenterol. 2019, 56, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sagias, F.G.; Mitry, R.R.; Hughes, R.D.; Lehec, S.C.; Patel, A.G.; Rela, M.; Mieli-Vergani, G.; Heaton, N.D.; Dhawan, A. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010, 19, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Tarzamani, M.K.; Farhang, S.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepat. Mon. 2010, 10, 12–16. [Google Scholar]
- Fulghesu, A.M.; Ciampelli, M.; Muzj, G.; Belosi, C.; Selvaggi, L.; Ayala, G.F.; Lanzone, A. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 1128–1135. [Google Scholar] [CrossRef]
- Vassilatou, E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J. Gastroenterol. 2014, 20, 8351–8363. [Google Scholar] [CrossRef]
- Uchida, D.; Takaki, A.; Adachi, T.; Okada, H. Beneficial and paradoxical roles of anti-oxidative nutritional support for non-alcoholic fatty liver disease. Nutrients 2018, 10, 977. [Google Scholar] [CrossRef] [Green Version]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Sohda, T.; Anan, A.; Fukunaga, A.; Takata, K.; Tanaka, T.; Yokoyama, K.; Morihara, D.; Takeyama, Y.; Shakado, S.; et al. Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Euroasian J. Hepato Gastroenterol. 2016, 6, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Marins, A.F.P.; Rabelo, A.L.R.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhães, F. Safety and efficacy of N-acetylcysteine in hospitalized patients with HIV-associated tuberculosis: An open-label, randomized, phase II trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Golec, K.; Czuczejko, J.; Tylzanowski, P.; Lecka, J. Strategies for modulating oxidative stress under diverse physiological and pathological conditions. Oxid. Med. Cell. Longev. 2018, 2018, 3987941. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef]
- Šalamon, S.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and dietary sses of n-acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Library of Medicine. ClinicalTrails.gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=n-acetyl+cysteine&cntry=&state=&city=&dist= (accessed on 30 November 2020).
- SBWIRE Global Acetylcysteine Market Size will Grow from US$ 490 Million to US$ 1650 Million by 2024. Available online: http://www.sbwire.com/press-releases/global-acetylcysteine-market-revenue-will-grow-at-223-cagr-to-2024-with-us-1650-million-market-size-1142001.htm (accessed on 30 November 2020).



| Author, Year | Experimental Model, NAC Dosage, and Intervention Period | Main Findings |
|---|---|---|
| Laurent et al., 2004 [65] | Male C57BL/6J obese ob/ob and lean wild-type mice between 8 and 10 weeks of age fed a standard diet were injected with NAC at 150 mg/kg every 3 days for 2 months. | NAC reduced lipoperoxidation and increased the intracellular and the mitochondrial pool of GSH, whereas the other antioxidant molecules tested were ineffective. However, it failed to ameliorate or lower cytochrome C release and caspase-3 activity. |
| de Oliveira et al., 2006 [67] | NAFLD was induced in ob/ob by MCD diet before oral gavage with SNAC 1.4 μmol/kg) for 4 weeks. | No significant changes in food intake or body weight were observed in comparison to the control group. After SNAC treatment, several genes belonging to oxidative phosphorylation, fatty acid biosynthesis, fatty acid metabolism and glutathione metabolism pathways were downregulated in comparison to the MCD group. |
| Oliveira et al., 2007 [68] | NAFLD was induced in male ob/ob mice using a MCD diet concomitantly with oral SNAC (1.4 mmol/kg) by gavage daily for 4 weeks. | SNAC markedly reduced liver steatosis, as well as parenchymal inflammation and microsomal triglyceride transfer protein. |
| de Oliveira et al., 2008 [66] | NAFLD was induced in male ob/ob mice by MCD diet and high-fat diets before the oral administration of SNAC solution (1.4 mg/kg/day) for 4 weeks. | SNAC inhibited the development of NAFLD, leading to a marked decrease in macro and microvacuolar steatosis and hepatic lipid peroxidation in the MCD group. SNAC treatment reversed the development of NAFLD in animals treated for 60 days with MCD and high-fat diets. |
| Chen et al., 2015 [70] | Polymerase η defiant (pol η−/−) mice fed HFD received NAC (1 mg/mL; wt/vol) or metformin (1.5 mg/mL; wt/vol) in drinking water, from week 8. | NAC and metformin suppressed DNA damage and the metabolic changes induced by a high-fat diet in both WT and pol η−/− mice, suggesting that metabolic abnormalities may be a general response to elevated DNA damage. |
| Gentric et al., 2015 [9] | Primary hepatocytes from ob/ob mice were treated with 7.5 mM NAC after seeding and during the whole culture time. | NAC treatment impaired the accumulation of ROS as well as that of Gpx3 and Hba1 mRNA, with no impact on cell viability. Moreover, the accumulation of BrdU at 60 h after plating was lower in ob/ob hepatocytes treated with NAC than in untreated cells, showing that treated cells progressed normally through the cell cycle. |
| Lee et al., 2015 [71] | Primary cultured hepatocytes from superoxide dismutase 1 (Sod1)-deficient and wild-type C57BL/6 mice treated with NAC at 2 mM for 2 h. | NAC was found to be effective in suppressing lipogenesis in the WT cells but not in the Sod1-KO cells. |
| Preziosi et al., 2017 [72] | Iron overload in liver-specific β-catenin knockout mice. NAC (2 g/kg) was added to drinking water for 3 months. | NAC protected KO +Fe from hepatic steatosis, injury and fibrosis, and prevented activation of AKT, ERK, NF-kB and reappearance of β-catenin. |
| Kumar et al., 2019 [69] | Primary hepatocytes from augmenter of liver regeneration (ALR) (ALR-L-knockout (KO)) mice pre-incubated with 10 mM NAC for 30 min. | NAC and recombinant ALR (rALR) both inhibited ALR depletion-induced miR-540 expression and lipid accumulation in hepatocytes. |
| Shin et al., 2019 [73] | Male catalase knockout (CKO) mice were fed an HFD for 6 weeks. NAC (60 mg/kg/day) or melatonin (500 μg/kg/day) were dissolved in saline solution and injected intraperitoneally for 6 weeks. | Co-treatment with NAC and melatonin suppressed fatty liver development and improved hepatic mitochondrial function. |
| Mai et al., 2020 [63] | NAC (10 μM) was applied in combination with MCD/lipopolysaccharide (LPS) or palmitate for 24 h in AML12 (alpha mouse liver 12) cells. | Thioredoxin interacting protein, Nod-like receptor family pyrin domain containing 3 (NLRP3), pro-caspase 1, and caspase-1 activity were suppressed by NAC. |
| Author, Year | Experimental Model, NAC Dosage, and Intervention Period | Main Findings |
|---|---|---|
| Fulghesu et al., 2002 [79] | Women with polycystic ovary syndrome (PCOS) received NAC at a dose of 1.8 g/day orally for 5–6 weeks. | NAC improved glucose tolerance and peripheral insulin sensitivity, but did not affect hepatic insulin extraction. |
| Pamuk et al., 2003 [74] | Patients with non-alcoholic steatohepatitis (NASH) received NAC at 1 g/day for 3 months. | NAC significantly improved liver function enzymes such as ALT, AST, and gamma-glutamyl transferase following a 4-week treatment period. |
| Mager et al., 2008 [75] | Two infants and 1 child with parenteral nutrition-induced liver disease received NAC intravenously, starting at a dose of 20 mg/kg/day and increasing to 10 mg/kg/day every 1 to 2 months. Maximum dosages of NAC of 70 mg/kg/day (patient 3) and 120 mg/kg/day (patient 2) were administered. | All of the patients studied demonstrated significant reductions in serum ferritin and in liver biochemistries when given NAC supplementation IV. In addition, red blood cell GSH levels returned to normal with NAC supplementation in 1 patient. |
| Khoshbaten et al., 2010 [78] | Patients with non-alcoholic fatty liver steatosis received NAC at 600 mg per 12 h. Patients were followed up using the same method of evaluation repeated in the first, second and third months. | NAC resulted in a significant decrease in serum ALT after three months. This effect was independent of the grade of steatosis in the initial diagnosis. |
| Sagias et al., 2010 [77] | Hepatocytes from steatotic donor livers were treated with NAC at 5 mM. | Addition of NAC during isolation of human hepatocytes from steatotic donor liver tissue significantly improved the outcome of cell isolation. |
| Oliveira et al., 2019 [76] | Patients with biopsy-proven NASH, treated with NAC (1.2 g), metformin (850–1500 mg/day) or NAC (1.2g) + metformin (850–1500 mg/day) for 48 weeks. | The combination of NAC and metformin performed better at improving the degree of steatosis, NAFLD activity score, and ALT levels at the end of the treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Shabalala, S.C.; Johnson, R.; Louw, J.; et al. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants 2020, 9, 1283. https://doi.org/10.3390/antiox9121283
Dludla PV, Nkambule BB, Mazibuko-Mbeje SE, Nyambuya TM, Marcheggiani F, Cirilli I, Ziqubu K, Shabalala SC, Johnson R, Louw J, et al. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants. 2020; 9(12):1283. https://doi.org/10.3390/antiox9121283
Chicago/Turabian StyleDludla, Phiwayinkosi V., Bongani B. Nkambule, Sithandiwe E. Mazibuko-Mbeje, Tawanda M. Nyambuya, Fabio Marcheggiani, Ilenia Cirilli, Khanyisani Ziqubu, Samukelisiwe C. Shabalala, Rabia Johnson, Johan Louw, and et al. 2020. "N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature" Antioxidants 9, no. 12: 1283. https://doi.org/10.3390/antiox9121283