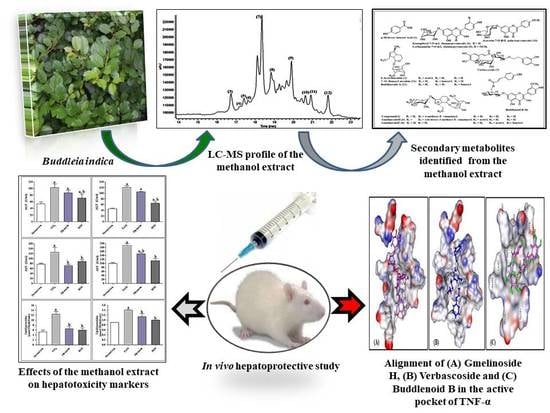
Metabolic Profiling of Buddleia indica Leaves using LC/MS and Evidence of their Antioxidant and Hepatoprotective Activity Using Different In Vitro and In Vivo Experimental Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of B. Indica Leaf Extract
2.3. LC-ESI-MS Profiling
2.3.1. HPLC Analysis
2.3.2. Mass Spectrometry
2.4. Biological Investigations
2.4.1. In Vitro Cytotoxicity
Cell Cultures
Cytotoxicity and Cell Viability Assay
2.4.2. Antioxidant and Hepatoprotective Activity
Chemical Reagents and Kits
In Vitro Antioxidant and Hepatoprotective Activity
Antioxidant Activity in HepG2 Cells
In Vivo Antioxidant and Hepatoprotective Assessment
Animals and Animal Treatment
LD50 Experiment
In Vivo Assessment of the Hepatoprotective Activity in CCl4 Induced Hepatotoxicity Model
Hepatoprotective Activity of BIM in the Tamoxifen Induced Hepatotoxicity Model
Evaluation of the Biochemical Parameters
Estimation of ALT and AST Activities
Estimation of Serum TAS
Estimation of Serum Catalase
Estimation of Serum SOD
Estimation of Tumor Necrosis Factor-Alpha (TNF-α)
Statistical Analysis
2.5. Molecular Modelling Studies
3. Results
3.1. LC-ESI-MS Analysis of BIM
3.2. Pharmacological Investigations
3.2.1. Evaluation of BIM Cytotoxic Activity
3.2.2. In Vitro Antioxidant and Hepatoprotective Activity
3.2.3. In Vivo Antioxidant and Hepatoprotective Activity in CCl4 Rat Model
3.2.4. In Vivo Antioxidant and Hepatoprotective Activity of BIM towards Tamoxifen Induced Hepatotoxicity
3.3. In Silico Molecular Modeling Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Schiff, E.R.; Sorrell, M.F.; Maddrey, W.C. Schiff’s Diseases of the Liver; Chapter 33 Drug-Induced Liver Disease; Chitturi, S., Farrell, G.C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Fathy, S.; Emam, M.; Agwa, S.A.; Zahra, F.A.; Youssef, F.; Sami, R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016, 62, 80–84. [Google Scholar] [PubMed]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Labib, R.M.; Eldahshan, O.A.; Singab, A.N. Synergistic hepatoprotective and antioxidant effect of Artichoke, Fig, Mulberry herbal mixture on HepG2 cells and their metabolic profiling Using NMR coupled with chemometrics. Chem. Biodivers. 2017, 14, e1700206. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.N.; Youssef, F.S.; Ashour, M.L.; Wink, M. The genus Eremophila (Scrophulariaceae): An ethnobotanical, biological and phytochemical review. J. Pharm. Pharmacol. 2013, 65, 1239–1279. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.R.; Delgadillo, A.J.; Hurtado, M.; Dominguez-Ramirez, A.M.; Medina, J.R.; Aoki, K. The antispasmodic activity of Buddleja scordioides and Buddleja perfoliata on isolated intestinal preparations. Biol. Pharm. Bull. 2006, 29, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; Wink, M. Morphological, anatomical, genetical and high performance thin layer chromatography profiling of Buddleia indica (Scrophulariaceae). Flora 2018, 246, 83–95. [Google Scholar] [CrossRef]
- Leeuwenberg, A.J.M. The Loganiaceae of Africa XVIII. Buddleja, L. II. Revision of the African and Asiatic Species. 1979. Available online: https://www.cabdirect.org/?target=%2fcabdirect%2fabstract%2f19800381419 (accessed on 1 August 2019).
- Martinez-Vazquez, M.; Ramirez Apan, T.O.; Aguilar, H.; Bye, R. Analgesic and antipyretic activities of an aqueous extract and of the flavone linarin of Buddleia cordata. Planta Med. 1996, 62, 137–140. [Google Scholar] [CrossRef]
- Houghton, P.J.; Mensah, A.Y.; Iessa, N.; Yong Hong, L. Terpenoids in Buddleja: Relevance to chemosystematics, chemical ecology and biological activity. Phytochemistry 2003, 64, 385–39313. [Google Scholar] [CrossRef]
- Piao, M.S.; Kim, M.R.; Lee, D.G.; Park, Y.; Hahm, K.S.; Moon, Y.H.; Woo, E.R. Antioxidative constituents from Buddleia officinalis. Arch. Pharm. Res. 2003, 26, 453–457. [Google Scholar]
- Zhang, Y.; Li, C.; Zhang, C.; Tao, B. Study on the chemical constituents of Buddleja purdomii. Zhong. Yao. Cai. 2005, 28, 994–995. [Google Scholar] [PubMed]
- Duschatzky, C.B.; Possetto, M.L.; Talarico, L.B.; Garcia, C.C.; Michis, F.; Almeida, N.V.; de Lampasona, M.P.; Schuff, C.; Damonte, E.B. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antivir. Chem. Chemother. 2005, 16, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.F.; Pereira, A.C.; Figueiredo, H.C.P.; Carvalho, D.A.; Silva, G.; Nunes, A.S.; Alves, D.S.; Carvalho, H.W.P. Antibacterial activity of plant extracts from Brazilian southeast region. Fitoterapia 2007, 78, 142–145. [Google Scholar] [CrossRef] [PubMed]
- El-Domiaty, M.M.; Wink, M.; Abdel Aal, M.M.; Abou-Hashem, M.M.; Abd-Alla, R.H. Antihepatotoxic activity and chemical constituents of Buddleja asiatica Lour. Z. Naturforsch. C 2009, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Bukhari, I.A.; Khan, R.A.; Shah, A.J.; Ahmad, I.; Malik, A. Presence of blood-pressure lowering and spasmolytic constituents in Buddleja crispa. Phytother. Res. 2009, 23, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Buddleja officinalis suppresses high glucose-induced vascular smooth muscle cell proliferation: Role of mitogen-activated protein kinases, nuclear factor-kappaB and matrix metalloproteinases. Exp. Biol. Med. 2010, 235, 247–255. [Google Scholar] [CrossRef]
- Tai, B.H.; Jung, B.Y.; Cuong, N.M.; Linh, P.T.; Tung, N.H.; Nhiem, N.X.; Huong, T.T.; Anh, N.T.; Kim, J.A.; Kim, S.K.; et al. Total peroxynitrite scavenging capacity of phenylethanoid and flavonoid glycosides from the flowers of Buddleja officinalis. Biol. Pharm. Bull. 2009, 32, 1952–1956. [Google Scholar] [CrossRef]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; El-Beshbishy, H.A.; Singab, A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018, 114, 302–310. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.B.; Wink, M. Eremophila maculata—Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine 2016, 23, 1484–1493. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytpther. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56. [Google Scholar] [CrossRef] [PubMed]
- Ellman, M. A spectrophotometric method for determination of reduced glutathione in tissues. Analyt. Biochem. 1959, 74, 214–226. [Google Scholar]
- Nishikimi, M.; Roa, N.; Yogi, K. Colorimetric determination of superoxide dismutase in tissue. Biochem. Biophys. Res. Common 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sayed, E.; Tolba, M.F.; Karonen, M. Antioxidant and hepatoprotective activities of flavonoids from Bauhinia hookeri. Rec. Nat. Prod. 2016, 10, 812–817. [Google Scholar]
- Ayoub, N.A.; Hashim, A.N.; Hussein, S.A.; Hegazi, N.M.; Hassanein, H.M.; Nawwar, M.A. Hepatoprotective effect of bay leaves crude extract on primary cultured rat hepatocytes. Eur. Sci. J. 2013, 9, 647–655. [Google Scholar]
- Youssef, F.S.; Ashour, M.L.; Ebada, S.S.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2017, 69, 733–742. [Google Scholar] [CrossRef]
- Bhandarkar, M.; Khan, A. Protective effect of Lawsona alba L. against CCl4 induced hepatic damage in albino rats. Ind. J. Exp. Biol. 2003, 4, 85–87. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Ōyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Protein Data Bank. Available online: http://www.pdb.org/ (accessed on 1 August 2019).
- Talaat, A.N.; Ebada, S.S.; Labib, R.M.; Esmat, A.; Youssef, F.S.; Singab, A.N.B. Verification of the anti-inflammatory activity of the polyphenolic-rich fraction of Araucaria bidwillii Hook. using phytohaemagglutinin-stimulated human peripheral blood mononuclear cells and virtual screening. J. Ethnopharmacol. 2018, 226, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crop. Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Fronti. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Panchal, M.A.; Murti, K.; Lambole, V. Pharmacological properties of Verbascum thapsus—A review. Int.J. Pharm. Sci. Rev. Res. 2010, 5, 73–77. [Google Scholar]
- Gong, Y.-L.; Pan, Q.-H.; Xiang, D.-X. Advances in studies on iridoids in plants of Scrophularia, L. and their bioactivity. Nat. Prod. Res. Develop. 2012, 24, 406–413. [Google Scholar]
- Ahmad, M.; Muhammad, N.; Jahan, N.; Ahmad, M.; Qureshi, M.; Jan, S.U. Spasmolytic effects of Scrophularia nodosa extract on isolated rabbit intestine. Pak. J. Pharm. Sci. 2012, 25, 267–275. [Google Scholar]
- Yamamoto, A.; Miyase, T.; Ueno, A.; Maeda, T. Buddlejasaponins I-IV, four new oleanane-triterpene saponins from the aerial parts of Buddleja japonica Hemsl. Chem. Pharma. Bull. 1991, 39, 2764–2766. [Google Scholar] [CrossRef]
- Hosny, M.; Rosazza, J.P. Gmelinosides A−L, twelve acylated iridoid glycosides from Gmelina a rborea. J. Nat. Prod. 1998, 61, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Yokokawa, Y. Two tyrosinase inhibiting flavonol glycosides from Buddleia coriacea. Phytochemistry 1992, 31, 1075–1077. [Google Scholar] [CrossRef]
- Avila, J.G.; de Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; de Vivar, A.R. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Harborne, J.B. Flavone and flavonol glycosides. In The Flavonoids: Advances in Research; Springer: Boston, MA, USA, 1982; pp. 261–311. [Google Scholar]
- Bileflimi, V.T.K. Chemical constituents of Verbascum, L. species. FABAD J. Pharm. Sci. 2004, 29, 93–107. [Google Scholar]
- Nicoletti, M.; Serafini, M.; Garbarino, J.A.; Gambaro, V. A chemosystematic study of Scrophulariaceae: Iridoid glycosides. Plant. Biosystem. 1988, 122, 13–24. [Google Scholar] [CrossRef]
- Sultana, N.; Akhter, M.; Khan, R.A.; Afza, N.; Tareen, R.B.; Malik, A. Nematicidal natural products from the aerial parts of Buddleja crispa. Nat. Prod. Res. 2010, 24, 783–788. [Google Scholar] [CrossRef]
- Pardo, F.; Perich, F.; Villarroel, L.; Torres, R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J. Ethnopharmacol. 1993, 39, 221–222. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef]
- Miyase, T.; Akahori, C.; Kohsaka, H.; Ueno, A. Acylated iridoid glycosides from Buddleja japonica HEMSL. Chem. Pharm. Bull. 1991, 39, 2944–2951. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Houghton, P.J.; Hoult, J. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J. Nat. Prod. 1999, 62, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Duff, R.; Bacon, J.; Mundie, C.; Farmer, V.; Russell, J.; Forrester, A. Catalpol and methylcatalpol: Naturally occurring glycosides in Plantago and Buddleia species. Biochem. J. 1965, 96, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Mensah, A.Y. Biologically Active Compounds from Buddleja Species, in Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Springer: Boston, MA, USA, 1999; pp. 343–368. [Google Scholar]
- Jamshed, F.; Ahmad, W.; Haque, A.E.; Saad, A.; Al-Jassabi, S. Ameliorative role of oleuropein extracted from olive leaf on tamoxifen-induced hepatic 8-hydroxydeoxyguanosine in DNA of Balb/C mice. World App. Sci. J. 2014, 30, 765–769. [Google Scholar]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. App. Pharmacol. 2010, 244, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mut. Res. Fund. Mol. Mech. Mut. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Briza: Pretoria, South Africa, 2017. [Google Scholar]





| No. | Rt (min) | UV (λmax) (nm) | (M-H)− m/z | Compounds | References |
|---|---|---|---|---|---|
| 1 | 1.36 | 248 | 136.95 | p-Hydroxy-benzoic acid | [41] |
| 2 | 1.62 | 252 | 387.13 | 6-Acetylaucubin | [42] |
| 3 | 16.37 | 242,330, | 430.79 | Kaempferol-7-o-α-l rhamnopyranoside | [43] |
| 4 | 17.15 | 244,292,330 | 667.55 | Catalpol-6-o-[4-methoxy-e-cinnamoyl-(3)-α -l-rhamnopyranoside | [44] |
| 5 | 17.32 | 244,306,330 | 901.62 | Gmelinoside H | [45] |
| 6 | 17.60 | 242,316 | 669.43 | Gmelinoside F | [45] |
| 7 | 18.47 | 242,330 | 623.41 | Verbascoside | [46] |
| 8 | 18.96 | 242,330 | 623.31 | Buddlenoid B | [47] |
| 9 | 19.93 | 244,330 | 461.29 | Isorhamnetin-7-o-α-L rhamnopyranoside | [48] |
| 10 | 20.47 | 244,330 | 445.15 | Acacetin-7-o-β-d galactoside | [49] |
| 11 | 20.95 | 244,300,328 | 449.06 | 2′-o-Benzoyl aucubin | [50] |
| 12 | 21.77 | 238,303 | 465.50 | Buddlejoside A | [51] |
| Cell Line | BIM (µg/mL) | Doxorubicin (µg/mL) |
|---|---|---|
| A549 | >1000 | 0.48 ± 0.031 |
| PC3 | 207.3 ± 19.00 | 0.49 ± 0.039 |
| HepG2 | 657.7 ± 56.01 | 0.22 ± 0.020 |
| Groups | AST a (U/mL) | ALT a (U/mL) | GSH b (mg/dL) | SOD c (U/mL) | TAC d (nmol/mL) |
|---|---|---|---|---|---|
| Control | 26.77 ± 1.68 * | 59.20 ± 0.95 * | 16.81 ± 0.08 * | 384.7 ± 13.50 * | 0.82 ± 0.016 * |
| CCl4 | 57.23 ± 1.87 | 85.36 ± 1.69 | 12.65 ± 0.29 | 251.0 ± 4.80 | 0.13 ± 0.014 |
| CCl4 + Silym (0.01 mg/mL) | 45.25 ± 0.82 * | 73.59 ± 1.06 * | 14.99 ± 0.06 * | 334.01 ± 6.41 * | 0.88 ± 0.010 * |
| CCl4 + Silym (0.1 mg/mL) | 41.57 ± 1.24 * | 67.09 ± 1.88 * | 16.59 ± 0.09 * | 384.01 ± 18.92 * | 0.99 ± 0.012 * |
| CCl4 + Silym (1 mg/mL) | 35.04 ± 1.54 * | 60.05 ± 1.75 * | 18.43 ± 0.17 * | 412.3 ± 3.11 * | 1.28 ± 0.033 * |
| CCl4 + BIM (0.01 mg/mL) | 57.10 ± 1.41 | 77.57 ± 0.81 * | 15.51 ± 0.71 * | 294.6 ± 12.40 | 0.90 ± 0.013 * |
| CCl4 + BIM (0.1 mg/mL) | 53.77 ± 2.36 | 73.02 ± 1.88 * | 15.93 ± 0.42 * | 326.8 ± 13.50 * | 1.09 ± 0.022 * |
| CCl4 + BIM (1 mg/mL) | 47.90 ± 0.42 * | 66.52 ± 1.53 * | 16.82± 0.55 * | 353.6 ± 8.20 * | 1.51 ± 0.028 * |
| Groups | * TAS (mmol/L) | * SOD (U/mL) | * CAT (U/mL) |
|---|---|---|---|
| Normal Control | 7.20 ± 0.63 b | 103.30 ± 1.7 b | 1.37 ± 0.21 b |
| CCl4 treated group | 2.61 ± 0.32 a | 41.81 ± 4.12 a | 0.39 ± 0.03 a |
| CCl4-treated rats + Silymarin | 5.31 ± 0.43 a,b | 84.8 ± 5.95 a,b | 0.88 ± 0.04 a,b |
| CCl4-treated rats + BIM | 5.61 ± 0.72 a,b | 97.2 ± 7.83 b | 1.12 ± 0.05 b |
| Groups | TNF-α (pg/g Protein) |
|---|---|
| Normal Control | 160 ± 11 b |
| TAM treated group | 700 ± 14 a |
| TAM-treated rats + Silymarin | 560 ±11 a,b |
| TAM-treated rats + BIM | 350 ± 10 a,b |
| Compound | pH-Based | Rule-Based |
|---|---|---|
| p-Hydroxy-benzoic acid (1) | −20.42 | −19.61 |
| 6-Acetylaucubin (2) | FD | −39.19 |
| Kaempferol-7-O-α-L rhamnopyranoside (3) | −40.38 | −38.34 |
| Catalpol-6-O-[4-methoxy-E -cinnamoyl-(3)-α -L-rhamnopyranoside (4) | −53.30 | −51.88 |
| GmelinosideH (5) | −59.66 | −62.58 |
| GmelinosideF (6) | −48.12 | −56.31 |
| Verbascoside (7) | −53.20 | −53.64 |
| Buddlenoid B (8) | −48.73 | −49.37 |
| Isorhamnetin-7-O-α-L rhamnopyranoside (9) | −37.74 | −40.56 |
| Acacetin-7-galactoside (10) | FD | FD |
| 2’-O-Benzoyl aucubin (11) | FD | −39.72 |
| Buddlejoside A (12) | FD | −35.88 |
| Ligand | −45.74 | −44.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Singab, A.N.B.; Wink, M. Metabolic Profiling of Buddleia indica Leaves using LC/MS and Evidence of their Antioxidant and Hepatoprotective Activity Using Different In Vitro and In Vivo Experimental Models. Antioxidants 2019, 8, 412. https://doi.org/10.3390/antiox8090412
Youssef FS, Ashour ML, El-Beshbishy HA, Singab ANB, Wink M. Metabolic Profiling of Buddleia indica Leaves using LC/MS and Evidence of their Antioxidant and Hepatoprotective Activity Using Different In Vitro and In Vivo Experimental Models. Antioxidants. 2019; 8(9):412. https://doi.org/10.3390/antiox8090412
Chicago/Turabian StyleYoussef, Fadia S., Mohamed L. Ashour, Hesham A. El-Beshbishy, Abdel Nasser B. Singab, and Michael Wink. 2019. "Metabolic Profiling of Buddleia indica Leaves using LC/MS and Evidence of their Antioxidant and Hepatoprotective Activity Using Different In Vitro and In Vivo Experimental Models" Antioxidants 8, no. 9: 412. https://doi.org/10.3390/antiox8090412