Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress
Abstract
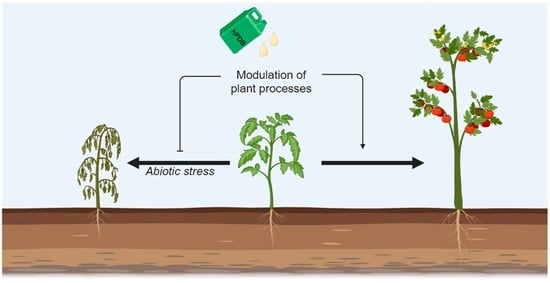
:1. Introduction
2. Climate Change and Plant Abiotic Stress
3. Plant Biostimulants: An Emerging Ecological Alternative
- (a)
- nutrient use efficiency,
- (b)
- tolerance to abiotic stress,
- (c)
- quality traits, or
- (d)
- availability of confined nutrients in the soil or rhizosphere.”
4. Higher Plant-Derived Biostimulants: Mechanisms of Action
4.1. Cellular and Molecular Levels
4.1.1. Plant Mineral Nutrition and Primary Metabolism
4.1.2. Specialized Metabolism
4.1.3. Photosynthetic Processes
4.1.4. Oxidative Metabolism
4.1.5. Signaling-Related Processes
4.2. Whole-Plant Level
4.2.1. Germination
4.2.2. Root Growth and Morphology
4.2.3. Shoot Growth and Morphology
4.2.4. Flowering
4.2.5. Fructification and Fruit Quality
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019; Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Gupta, S.; Bhattacharyya, P.; Kulkarni, M.G.; Doležal, K. Editorial: Growth Regulators and Biostimulants: Upcoming Opportunities. Front. Plant Sci. 2023, 14, 1209499. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. The European Green Deal. In Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Liu, Y.; Ishaq, M.; Shah, T.; Abdullah; Ilyas, A.; Din, I.U. Climate Change and Its Impact on the Yield of Major Food Crops: Evidence from Pakistan. Foods 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D. Can Biostimulants Be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; R, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of Biostimulants in Mitigating the Effects of Climate Change on Crop Performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- Akbari, M.; Najafi Alamdarlo, H.; Mosavi, S.H. The Effects of Climate Change and Groundwater Salinity on Farmers’ Income Risk. Ecol. Indic. 2020, 110, 105893. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Martí-Guillen, J.M.; Martínez-Lorente, S.E.; Rivero, R.M. ROS and NO Phytomelatonin-Induced Signaling Mechanisms under Metal Toxicity in Plants: A Review. Antioxidants 2021, 10, 775. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Pardo-Hernández, M.; Martí-Guillén, J.M.; López-Delacalle, M.; Rivero, R.M. Interaction between Melatonin and NO: Action Mechanisms, Main Targets, and Putative Roles of the Emerging Molecule NOmela. Int. J. Mol. Sci. 2022, 23, 6646. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitro-Oxidative Stress vs Oxidative or Nitrosative Stress in Higher Plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Martí-Guillén, J.M.; Pardo-Hernández, M.; Martínez-Lorente, S.E.; Almagro, L.; Rivero, R.M. Redox Post-Translational Modifications and Their Interplay in Plant Abiotic Stress Tolerance. Front. Plant Sci. 2022, 13, 1027730. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, K.; Akhtar, M.A.; Maqbool, M.A. Salinity Stress in Crop Plants: Effects of Stress, Tolerance Mechanisms and Breeding Strategies for Improvement. J. Agric. Basic Sci. 2017, 2, 70–85. [Google Scholar]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Challabathula, D.; Analin, B.; Mohanan, A.; Bakka, K. Differential Modulation of Photosynthesis, ROS and Antioxidant Enzyme Activities in Stress-Sensitive and -Tolerant Rice Cultivars during Salinity and Drought upon Restriction of COX and AOX Pathways of Mitochondrial Oxidative Electron Transport. J. Plant Physiol. 2022, 268, 153583. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Russo, R.O.; Berlyn, G.P. The Use of Organic Biostimulants to Help Low Input Sustainable Agriculture. J. Sustain. Agric. 1991, 1, 19–42. [Google Scholar] [CrossRef]
- Russo, R.O.; Berlyn, G.P. Vitamin-Humic-Algal Root Biostimulant Increases Yield of Green Bean. HortScience 1992, 27, 847. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: A New Paradigm for the Sustainable Intensification of Crops. In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: London, UK, 2020; ISBN 978-1-00-304786-5. [Google Scholar]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- La Torre, A.; Battaglia, V.; Caradonia, F. An Overview of the Current Plant Biostimulant Legislations in Different European Member States. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Mącik, M.; Gryta, A.; Frąc, M. Chapter Two—Biofertilizers in Agriculture: An Overview on Concepts, Strategies and Effects on Soil Microorganisms. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 162, pp. 31–87. [Google Scholar]
- Qi, X.; Li, K.; Chen, L.; Zhang, Y.; Zhang, N.; Gao, W.; Li, Y.; Liu, X.; Fan, Z. Plant Defense Responses to a Novel Plant Elicitor Candidate LY5-24-2. Int. J. Mol. Sci. 2022, 23, 5348. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Ahmad, A.; Blasco, B.; Martos, V. Combating Salinity Through Natural Plant Extracts Based Biostimulants: A Review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the Environmental Impact of Chemically-Produced Protein Hydrolysate from Leather Waste vs. Enzymatically-Produced Protein Hydrolysate from Legume Grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants From Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 626301. [Google Scholar] [CrossRef]
- Brazales-Cevallos, D.K.; Romero-Contreras, Y.J.; Vences-Guzmán, M.Á.; Torres, M.; Aviles-Baltazar, N.Y.; Sohlenkamp, C.; Serrano, M. Transcriptional Characterization of the Biostimulant Effect of Moringa Oleifera Leaf Extracts Using Arabidopsis Thaliana as a Model. S. Afr. J. Bot. 2022, 144, 250–256. [Google Scholar] [CrossRef]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Plant-Derived Biostimulants on White Head Cabbage Seedlings Grown under Controlled Conditions. Sustainability 2019, 11, 5317. [Google Scholar] [CrossRef]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica oleracea L. Var. capitata L.) Seedlings Grown under Controlled Conditions. Sustainability 2020, 12, 1871. [Google Scholar] [CrossRef]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.B.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed Priming with Sorghum Water Extract Improves the Performance of Camelina (Camelina sativa (L.) Crantz.) under Salt Stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Wise, K.; Selby-Pham, J.; Chai, X.; Simovich, T.; Gupta, S.; Gill, H. Fertiliser Supplementation with a Biostimulant Complex of Fish Hydrolysate, Aloe Vera Extract, and Kelp Alters Cannabis Root Architecture to Enhance Nutrient Uptake. Sci. Hortic. 2024, 323, 112483. [Google Scholar] [CrossRef]
- Cirillo, C.; Rouphael, Y.; Pannico, A.; El-Nakhel, C.; Colla, G.; De Pascale, S. Application of Protein Hydrolysate-Based Biostimulant as New Approach to Improve Performance of Bedding Plants. In Proceedings of the ISHS Acta Horticulturae 1215: International Symposium on Greener Cities for More Efficient Ecosystem Services in a Climate Changing World, Bologna, Italy, 12–15 September 2017; pp. 443–448. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Mozzetti Monterumici, C.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of Bean Plants with Tomato Plants Hydrolysates. Effect on Biomass Production, Chlorophyll Content and N Assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of Salt Stress Effects on the Growth, Physio-Chemical Attributes and Yields of Phaseolus vulgaris L. Plants by the Combined Application of Salicylic Acid and Moringa Oleifera Leaf Extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Howladar, S.M. A Novel Moringa Oleifera Leaf Extract Can Mitigate the Stress Effects of Salinity and Cadmium in Bean (Phaseolus vulgaris L.) Plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Varma, C.B.; Howladar, S.M. Common Bean (Phaseolus vulgaris L.) Seedlings Overcome NaCl Stress as a Result of Presoaking in Moringa Oleifera Leaf Extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous Applications of Moringa Leaf Extract Effect on Retrotransposon, Ultrastructural and Biochemical Contents of Common Bean Plants under Environmental Stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.-S.M. Maize (Zea mays L.) Grains Extract Mitigates the Deleterious Effects of Salt Stress on Common Bean (Phaseolus vulgaris L.) Growth and Physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.-S.M.; Elrys, A.S.; Boghdady, M.S. Can Licorice Root Extract Be Used as an Effective Natural Biostimulant for Salt-Stressed Common Bean Plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Al-Araby, A.A.; Xuan, T.D. Growth Traits, Physiological Parameters and Hormonal Status of Snap Bean (Phaseolus vulgaris L.) Sprayed with Garlic Cloves Extract. Arch. Agron. Soil Sci. 2018, 64, 1068–1082. [Google Scholar] [CrossRef]
- Mkindi, A.G.; Tembo, Y.L.B.; Mbega, E.R.; Smith, A.K.; Farrell, I.W.; Ndakidemi, P.A.; Stevenson, P.C.; Belmain, S.R. Extracts of Common Pesticidal Plants Increase Plant Growth and Yield in Common Bean Plants. Plants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; EL-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and Ammi Seed Extracts Modulate Antioxidant Defence System and Alleviate Salinity Stress in Cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Abbas, W.; Ashraf, M.; Akram, N.A. Alleviation of Salt-Induced Adverse Effects in Eggplant (Solanum melongena L.) by Glycinebetaine and Sugarbeet Extracts. Sci. Hortic. 2010, 125, 188–195. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Ganugi, P.; Fiorini, A.; Miras Moreno, B.; Zhang, L.; Cardarelli, M.; et al. Copper Boosts the Biostimulant Activity of a Vegetal-Derived Protein Hydrolysate in Basil: Morpho-Physiological and Metabolomics Insights. Front. Plant Sci. 2023, 14, 1235686. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.A.H.; Badran, F.S.; Ahmed, E.T.; Taha, R.A.; Abdel-Mola, M.A.M. Effect of Compost and some Natural Stimulant Treatments on: Ii. Corms Production and Chemical Constituents of (Gladiolus grandiflorus cv. Peter Pears) Plants. Sci. J. Flowers Ornam. Plants 2018, 5, 115–126. [Google Scholar] [CrossRef]
- Younis, A.; Akhtar, M.S.; Riaz, A.; Zulfiqar, F.; Qasim, M.; Farooq, A.; Tariq, U.; Ahsan, M.; Bhatti, Z.M. Improved Cut Flower and Corm Production by Exogenous Moringa Leaf Extract Application on Gladiolus Cultivars. Acta Sci. Pol. Hortorum Cultus 2018, 17, 25–38. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Garde-Cerdán, T.; Zalacain, A.; Garcia, R.; Cabrita, M.J.; Salinas, M.R. Vine-Shoot Waste Aqueous Extract Applied as Foliar Fertilizer to Grapevines: Effect on Amino Acids and Fermentative Volatile Content. Food Chem. 2016, 197, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Moscatel Vine-Shoot Extracts as a Grapevine Biostimulant to Enhance Wine Quality. Food Res. Int. 2017, 98, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Torregrosa, L.; Zalacain, A.; Ojeda, H.; Bouckenooghe, V.; Schneider, R.; Alonso, G.L.; Salinas, M.R. The Microvine, a Plant Model to Study the Effect of Vine-Shoot Extract on the Accumulation of Glycosylated Aroma Precursors in Grapes. J. Sci. Food Agric. 2018, 98, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein Hydrolysates Modulate Leaf Proteome and Metabolome in Water-Stressed Grapevines. Sci. Hortic. 2020, 270, 109413. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein Hydrolysates Effects on Grapevine (Vitis vinifera L., cv. Corvina) Performance and Water Stress Tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak Extract Application to Grapevines as a Plant Biostimulant to Increase Wine Polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; García-Martínez, A.; González-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic Vegetable Extract with Bio- Active Components: Influence of Fertiliser on the Colour and Anthocyanins of Red Grapes. J. Sci. Food Agric. 2007, 87, 2310–2318. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. Growth and Nutraceutical Properties Are Enhanced by Biostimulants in a Long-Term Period: Chemical and Metabolomic Approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Nasir, M.; Khan, A.S.; Basra, S.M.A.; Malik, A.U. Foliar Application of Moringa Leaf Extract, Potassium and Zinc Influence Yield and Fruit Quality of ‘Kinnow’ Mandarin. Sci. Hortic. 2016, 210, 227–235. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Sorrentino, M.; Panzarová, K.; Spyroglou, I.; Spíchal, L.; Buffagni, V.; Ganugi, P.; Rouphael, Y.; Colla, G.; Lucini, L.; De Diego, N. Integration of Phenomics and Metabolomics Datasets Reveals Different Mode of Action of Biostimulants Based on Protein Hydrolysates in Lactuca sativa L. and Solanum lycopersicum L. under Salinity. Front. Plant Sci. 2022, 12, 808711. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant Biostimulants from Seaweeds or Vegetal Proteins Enhance the Salinity Tolerance in Greenhouse Lettuce by Modulating Plant Metabolism in a Distinctive Manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-Based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Švecová, E.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a Plant-Derived Protein Hydrolysate to Improve Crop Performances under Different Growing Conditions. Acta Hortic. 2013, 1009, 175–179. [Google Scholar] [CrossRef]
- Shalaby, O.A. Moringa Leaf Extract Increases Tolerance to Salt Stress, Promotes Growth, Increases Yield, and Reduces Nitrate Concentration in Lettuce Plants. Sci. Hortic. 2024, 325, 112654. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R.; et al. Evaluation of Borage Extracts As Potential Biostimulant Using a Phenomic, Agronomic, Physiological, and Biochemical Approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef]
- De Lucia, B. Type of Bio-Stimulant and Application Method Effects on Stem Quality and Root System Growth in L.A. Lily. Eur. J. Hortic. Sci. 2012, 77, 10. [Google Scholar]
- Mutlu-Durak, H.; Yildiz Kutman, B. Seed Treatment with Biostimulants Extracted from Weeping Willow (Salix Babylonica) Enhances Early Maize Growth. Plants 2021, 10, 1449. [Google Scholar] [CrossRef]
- Pehlivan, N. Salt Stress Relief Potency of Whortleberry Extract Biopriming in Maize. 3 Biotech 2018, 8, 89. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Tolisano, C.; Luzi, F.; Regni, L.; Proietti, P.; Puglia, D.; Gigliotti, G.; Di Michele, A.; Priolo, D.; Del Buono, D. A Way to Valorize Pomace from Olive Oil Production: Lignin Nanoparticles to Biostimulate Maize Plants. Environ. Technol. Innov. 2023, 31, 103216. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological Activity of Vegetal Extracts Containing Phenols on Plant Metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Senizza, B.; Regni, L.; Tolisano, C.; Proietti, P.; Trevisan, M.; Lucini, L.; Rouphael, Y.; Del Buono, D. Biochemical Insights into the Ability of Lemna minor L. Extract to Counteract Copper Toxicity in Maize. Plants 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-Containing Organic Substances Stimulate Phenylpropanoid Metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Mostofa, M.G.; Rahman, M.d.M.; Abdel-Farid, I.B.; Tran, L.-S.P. Extracts from Yeast and Carrot Roots Enhance Maize Performance under Seawater-Induced Salt Stress by Altering Physio-Biochemical Characteristics of Stressed Plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an Alfalfa Protein Hydrolysate on the Gene Expression and Activity of Enzymes of the Tricarboxylic Acid (TCA) Cycle and Nitrogen Metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant Activity of Two Protein Hydrolyzates in the Growth and Nitrogen Metabolism of Maize Seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa Plant-Derived Biostimulant Stimulate Short-Term Growth of Salt Stressed Zea mays L. Plants. Plant Soil 2012, 364, 145–158. [Google Scholar] [CrossRef]
- El-Sharony, T.F.; El-Gioushy, S.; Oa, A. Effect of Foliar Application with Algae and Plant Extracts on Growth, Yield and Fruit Quality of Fruitful Mango Trees Cv. Fagri Kalan. J. Hortic. 2015, 2, 162. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Shaukat, K.; Hafeez, M.B.; Batool, A.; Hasanuzzaman, M. Oxidative Stress Tolerance Potential of Milk Thistle Ecotypes after Supplementation of Different Plant Growth-Promoting Agents under Salinity. Plant Physiol. Biochem. 2021, 166, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Suryaman, M.; Sunarya, Y.; Istarimila, I.; Fudholi, A. Effect of Salinity Stress on the Growth and Yield of Mungbean (Vigna radiata (L.) R. Wilczek) Treated with Mangosteen Pericarp Extract. Biocatal. Agric. Biotechnol. 2021, 36, 102132. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kothari, R.M. Recycled Cereal Proteins as Foliar Spray Enhances Quality and Production of Food Crops. Resour. Conserv. Recycl. 1993, 9, 213–221. [Google Scholar] [CrossRef]
- Habib, N.; Ashraf, M.; Ali, Q.; Perveen, R. Response of Salt Stressed Okra (Abelmoschus Esculentus Moench) Plants to Foliar-Applied Glycine Betaine and Glycine Betaine Containing Sugarbeet Extract. S. Afr. J. Bot. 2012, 83, 151–158. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Miras-Moreno, B.; Senizza, B.; Lucini, L.; Trevisan, M.; Morelli Venturi, D.; Costantino, F.; Proietti, P. Biostimulant Effects of an Aqueous Extract of Duckweed (Lemna minor L.) on Physiological and Biochemical Traits in the Olive Tree. Agriculture 2021, 11, 1299. [Google Scholar] [CrossRef]
- Lorenzo, P.; Souza-Alonso, P.; Guisande-Collazo, A.; Freitas, H. Influence of Acacia Dealbata Link Bark Extracts on the Growth of Allium cepa L. Plants under High Salinity Conditions. J. Sci. Food Agric. 2019, 99, 4072–4081. [Google Scholar] [CrossRef]
- Ghezal, N.; Rinez, I.; Sbai, H.; Saad, I.; Farooq, M.; Rinez, A.; Zribi, I.; Haouala, R. Improvement of Pisum Sativum Salt Stress Tolerance by Bio-Priming Their Seeds Using Typha Angustifolia Leaves Aqueous Extract. S. Afr. J. Bot. 2016, 105, 240–250. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A. Using Moringa Oleifera Extract as Biostimulant Enhancing the Growth, Yield and Nutrients Accumulation of Pea Plants. J. Plant Nutr. 2018, 41, 425–431. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; ElSayed, A.I.; Merwad, A.-R.M.A.; Rady, M.M. Stimulating Antioxidant Defenses, Antioxidant Gene Expression, and Salt Tolerance in Pisum Sativum Seedling by Pretreatment Using Licorice Root Extract (LRE) as an Organic Biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-Based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein Hydrolysate or Plant Extract-Based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef]
- Elrys, A.S.; Merwad, A.-R.M.A.; Abdo, A.I.E.; Abdel-Fatah, M.K.; Desoky, E.-S.M. Does the Application of Silicon and Moringa Seed Extract Reduce Heavy Metals Toxicity in Potato Tubers Treated with Phosphate Fertilizers? Environ. Sci. Pollut. Res. 2018, 25, 16776–16787. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Olivera, V.; Morales-Payan, J.P.; Robles, W.; Goenaga, R. Effects of Biostimulants on Fruits of Pulasan [Nephelium Ramboutan-Ake (Labillardière) Leenhouts]. Acta Hortic. 2014, 1042, 101–104. [Google Scholar] [CrossRef]
- Ashraf, R.; Sultana, B.; Iqbal, M.; Mushtaq, M. Variation in Biochemical and Antioxidant Attributes of Raphanus Sativus in Response to Foliar Application of Plant Leaf Extracts as Plant Growth Regulator. J. Genet. Eng. Biotechnol. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M. The Potential of Moringa Oleifera Extract as a Biostimulant in Enhancing the Growth, Biochemical and Hormonal Contents in Rocket (Eruca vesicaria subsp. sativa) Plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the Growth, Yield and Volatile Oil Content of Pelargonium graveolens L. Herit by Foliar Application with Moringa Leaf Extract through Motivating Physiological and Biochemical Parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa Leaf Extract as Biostimulant Improves Water Use Efficiency, Physio-Biochemical Attributes of Squash Plants under Deficit Irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-Based Biostimulant as Sustainable Alternative to Synthetic Growth Regulators in Two Sweet Cherry Cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of Natural Biostimulants on Yield and Nutritional Quality: An Example of Sweet Yellow Pepper (Capsicum annuum L.) Plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Aluko, M. ARPN Journal of Agricultural and Biological Science Moringa Leaf Extract on the Growth and Yield of Pepper (Capsicum annuum L.). ARPN J. Agric. Biol. Sci. 2016, 11, 107–109. [Google Scholar]
- Desoky, E.-S.M.; Elrys, A.S.; Rady, M.M. Integrative Moringa and Licorice Extracts Application Improves Capsicum annuum Fruit Yield and Declines Its Contaminant Contents on a Heavy Metals-Contaminated Saline Soil. Ecotoxicol. Environ. Saf. 2019, 169, 50–60. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and Nutritional Quality of Vesuvian Piennolo Tomato PDO as Affected by Farming System and Biostimulant Application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled With N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Sadasivam Vinoth, S.V.; Sundari, S.; Packiaraj Gurusaravanan, P.G.; Subiramani Sivakumar, S.S.; Govindarajan Siva, G.S.; Kumar, G.P.; Manju, M.; Velmurugan, K.; Lakshminarayana, V.; Jayabalan, N. Evaluation of Seagrass Liquid Extract on Salt Stress Alleviation in Tomato Plants. Asian J. Plant Sci. 2017, 16, 172–183. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous Garlic Extract Stimulates Growth and Antioxidant Enzymes Activity of Tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from Food Processing By-Products: Agronomic, Quality and Metabolic Impacts on Organic Tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; ur Rehman, H. Supplementing Organic Biostimulants into Growing Media Enhances Growth and Nutrient Uptake of Tomato Transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Farooq, M.; Nawaz, A. Seed Priming with Sorghum Extracts and Benzyl Aminopurine Improves the Tolerance against Salt Stress in Wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2018, 24, 239–249. [Google Scholar] [CrossRef]
- Haider, F.; Bagchi, G.D.; Singh, A.K. Effect of Calliterpenone on Growth, Herb Yield and Oil Quality of Mentha Arvensis. Int. J. Integr. Biol. 2009, 7, 53–57. [Google Scholar]
- Matsumiya, Y.; Kubo, M.; Matsumiya, Y.; Kubo, M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean. In Soybean and Nutrition; IntechOpen: Shanghai, China, 2011; ISBN 978-953-307-536-5. [Google Scholar]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a Carob Enzymatic Extract: Potential Use as a Biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Ganie, S.A.; Hossain, M.S.; Gomaa, A.M. Seed Priming with Cypress Leaf Extract Enhances Photosynthesis and Antioxidative Defense in Zucchini Seedlings under Salt Stress. Sci. Hortic. 2022, 293, 110707. [Google Scholar] [CrossRef]
- De Diego, N.; Spíchal, L. Presence and Future of Plant Phenotyping Approaches in Biostimulant Research and Development. J. Exp. Bot. 2022, 73, 5199–5212. [Google Scholar] [CrossRef]
- Ertani, L.L. Begoña Miras-Moreno, Andrea Bioactive Compounds and Evaluation of Biostimulant Activity. In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: London, UK, 2020; ISBN 978-1-00-304786-5. [Google Scholar]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Chaabani, S.; Roula, R.; Muscolo, A. Bio-Priming Mitigates Detrimental Effects of Salinity on Maize Improving Antioxidant Defense and Preserving Photosynthetic Efficiency. Plant Physiol. Biochem. 2018, 132, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Guy, C.L. Beta-Amylase Induction and the Protective Role of Maltose during Temperature Shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Kochhar, S.L.; Gujral, S.K. Secondary Plant Metabolites. In Plant Physiology: Theory and Applications; Kochhar, S.L., Gujral, S.K., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 590–610. ISBN 978-1-108-48639-2. [Google Scholar]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Chapter 9—Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Thorston, UK, 2019; pp. 157–168. ISBN 978-0-12-816451-8. [Google Scholar]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Chapter 12—Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 271–291. ISBN 978-0-12-800876-8. [Google Scholar]
- del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as Growth Regulators During Abiotic Stress Tolerance in Plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant Mode of Action. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 205–227. ISBN 978-1-119-35725-4. [Google Scholar]
- Gilroy, S.; Jones, D.L. Through Form to Function: Root Hair Development and Nutrient Uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R. Legume Seeds as an Important Component of Human Diet. Foods 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]



| Crop | hPDB | Application Method | Ref. |
|---|---|---|---|
| Arabidopsis (Arabidopsis thaliana) | Moringa (Moringa oleifera) leaf extract, Trainer®, Vegamin®, and PHs from Fabaceae, Malvaceae, Brassicaceae, Solanaceae, and Graminaceae | Seed priming | [37,38] |
| Cabbage (Brassica oleracea) | Extracts from mugwort (Artemisia vulgaris), calendula (Calendula officinalis) flowers, purple coneflower (Echinacea purpurea) flowers and leaves, chamomile (Matricaria chamomilla) flowers, basil (Ocimum basilicum), giant goldenrod (Solidago gigantea) leaves, comfrey (Symphytum officinale) root, dandelion (Taraxacum officinale) flowers, leaves, and root, valerian (Valeriana officinalis) root, aloe vera (Aloe vera) leaves, chokeberry (Aronia melanocarpa) fruits, red beet (Beta vulgaris) root, horsetail (Equisetum arvense), common sea buckthorn (Hippophae rhamnoides) fruits, hypericum (Hypericum perforatum), red lentil (Lens culinaris) seeds, common bracken (Pteridium aquilinum) leaves, knotgrass (Polygonum aviculare), pea (Pisum sativum) seeds, broadleaf plantain (Plantago major), red clover (Trifolium pratense) flowers, and nettle (Urtica dioica) leaves and root | Foliar spray | [39,40] |
| Camelina (Camelina sativa) | Shorgum (Shorgum sp.) water extract | Seed priming | [41] |
| Cannabis (Cannabis sativa) | Aloe vera (Aloe vera) extract and aloe vera, fish, and kelp complex | Nutrient solution | [42] |
| Cascading geranium (Pelargonium peltatum) | Trainer® | Foliar spray | [43] |
| Common bean (Phaseolus vulgaris) | Extracts from fish bean (Tephrosia vogelii) and tree marigold (Tithonia diversifolia), garlic (Allium sativum) cloves, licorice (Glycirrhiza glabra) root, moringa (Moringa oleifera) leaves, and tomato (Solanum lycopersicum) powder hydrolysate. | Foliar spray, seed priming, and substrate application | [44,45,46,47,48,49,50,51,52] |
| Cowpea (Vigna unguiculata) | Extracts from fennel (Foeniculum vulgare) and ammi (Ammi visnaga) seeds | Foliar spray | [53] |
| Eggplant (Solanum melongena) | Sugarbeet (Beta vulgaris) extract | Foliar spray | [54] |
| Genovese basil (Ocimum basilicum) | Trainer® | Foliar spray | [55] |
| Gladiolus (Gladiolus grandiflorus) | Extracts from moringa (Moringa oleifera) leaves, garlic (Allium sativum) extract, and licorice (Glycyrrhiza glabra) root | Foliar spray | [56,57] |
| Grapevine (Vitis vinifera) | Trainer®, Stimtide®, and soybean (Glycine max) hydrolysate and extracts from french oak (Quercus sessiliflora), vine-shoot (Vitis vinifera) waste, and carob germ (Ceratonia silique) and from mixture of maize (Zea mays) and sorghum (Sorghum sp.) distiller’s dried grains. | Foliar spray and substrate application | [58,59,60,61,62,63,64] |
| Habanero pepper (Capsicum chinensis) | Red grape (Vitis vinifera) skin extract and alfafa (Medicago sativa) hydrolysate | Foliar spray | [65] |
| Kinnow mandarin (Citrus nobilis × Citrus deliciosa) | Moringa (Moringa oleifera) leaf extract | Foliar spray | [66] |
| Lamb’s lettuce (Valerianella locusta) | Trainer® | Foliar spray | [67] |
| Lettuce (Lactuca sativa) | Auxym®, LISIVEG®, Trainer®, Vegamin®, and PHs from Fabaceae, Malvaceae, Brassicaceae, Solanaceae, and Graminaceae. Extracts from moringa (Moringa oleifera) leaves and leaves and flowers of borage (Borago officinalis). | Foliar spray, seed priming, root application, and substrate application before transplant. | [68,69,70,71,72,73,74,75] |
| Lilium ‘Brindisi’ (Lilium longiflorum × Lilium x elegans) | Alfalfa (Medicago sativa) hydrolysate | Foliar spray and substrate application | [76] |
| Maize (Zea mays) | Lignin nanoparticles from olive (Olea europaea) waste, Trainer®, hydrolysates from alfalfa (Medicago sativa), dry apple (Malus domestica), and extracts from carrot (Daucus carota) root, blueberry (Vaccinium corymbosum) fruits, duckweed (Lemna minor), hawthorn (Crataegus monogina) leaves, common grapevine (Vitis vinifera) grape skin, rosemary (Rosmarinus officinalis), white wormwood (Artemisia herba-alba), whortleberry (Vaccinium arctostaphylos) fruit, and willow tree (Salix babylonica) barks and leaves | Foliar spray, nutrient solution, seed priming, and substrate application | [72,77,78,79,80,81,82,83,84,85,86,87] |
| Mango (Mangifera indica) | Roselle (Hibischus sabdariffa), garlic (Allium sativum) clove, and algae extracts alone or combinated | Foliar spray | [88] |
| Milk thistle (Silybum marianum) | Moringa (Moringa oleifera) leaf extract | Substrate application | [89] |
| Mung bean (Vigna radiata) | Cereal PHs and angosteen (Garcinia mangostana) pericarp extract | Foliar spray and seed priming | [90,91] |
| Okra (Abelmoschus esculentus) | Sugarbeet (Beta vulgaris) extract | Foliar spray | [92] |
| Olive (Olea europaea) tree | Duckweed (Lemna minor) plant extract | Foliar spray | [93] |
| Onion (Allium cepa) | Mimosa (Acacia dealbata) bark extract | Foliar spray | [94] |
| Pea (Pisum sativum) | Trainer® and extracts from licorice (Glycyrrhiza glabra) root, moringa (Moringa oleifera) leaves, and narrow-leaf cattail (Typha angustifolia) leaves | Foliar spray, seed priming, and shoot application | [79,95,96,97] |
| Perennial wall rocket (Diplotaxis teniufolia) | Auxym®, Trainer®, and their combination | Foliar spray | [98,99] |
| Potato (Solanum tuberosum) | Moringa (Moringa oleifera) seed extract | Substrate application | [100] |
| Pulasan (Nephelium ramboutan-ake) | ComCat® | Foliar spray | [101] |
| Radish (Raphanus sativus) | Extracts from leaves of mulberry (Morus nigra), brassica (Brassica napus), sorghum (Sorghum bicolor), and moringa (Moringa oleifera) | Foliar spray | [102] |
| Rice (Oryza sativa) | Cereal PHs | Foliar spray | [91] |
| Rocket (Eruca vesicaria) | Moringa (Moringa oleifera) leaf and twig extracts | Foliar spray | [103] |
| Rose-scented geranium (Pelargonium graveolens) | Moringa (Moringa oleifera) leaf extract | Foliar spray | [104] |
| Spinach (Spinacia oleracea) | Amalgerol® and Trainer® | Foliar spray | [67,105,106] |
| Squash (Curcubita pepo) | Moringa (Moringa oleifera) leaf extract | Foliar spray | [107] |
| Sweet cherry (Prunus avium) | Auxym® | Foliar spray | [108] |
| Sweet pepper (Capsicum annuum) | Radifarm®, Megafol®, Viva®, and Benefit® and extracts from moringa (Moringa oleifera) seeds and leaves and licorice (Glycyrrhiza glabra) root | Foliar spray, nutrient solution, and substrate application | [109,110,111] |
| Tomato (Solanum lycopersicum) | Auxym®, Trainer®, Vegamin®, PHs from alfalfa (Medicago sativa), Fabaceae, Malvaceae, Brassicaceae, Solanaceae, and Graminaceae and extracts from crushed maize (Zea mays) grain, garlic (Allium sativum) cloves, seagrass (Zostera marina), barley (Hordeum vulgare), and grain waste and processing residues from fennel (Foeniculum vulgare) and lemon (Citrus limon) | Foliar spray, seed priming, nutrient solution, cutting immersion, and substrate application | [68,79,112,113,114,115,116,117,118,119,120,121,122,123] |
| Wheat (Triticum aestivum) | Cereal PHs and shorgum (Shorgum sp.) water extract | Foliar spray and seed priming | [91,124] |
| White rocket (Diplotaxis erucoides) | Auxym® and Trainer® | Foliar spray | [6] |
| Wild mint (Mentha arvensis) | Calliterpenone from large-leaf beauty berry (Callicarpa macrophylla) extract | Sucker immersion | [125] |
| Wild mustard (Brassica rapa) | Soybean (Glycine max) waste hydrolysate | Root application | [126] |
| Wild tomato (Solanum pimpinellifolium) | Carob (Ceratonia siliqua) germ hydrolysate extract | Substrate application | [127] |
| Zucchini (Curcubita pepo) | Cypress (Cupressus macrocarpa) leaf extract | Seed priming | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. https://doi.org/10.3390/antiox13030318
Martínez-Lorente SE, Martí-Guillén JM, Pedreño MÁ, Almagro L, Sabater-Jara AB. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants. 2024; 13(3):318. https://doi.org/10.3390/antiox13030318
Chicago/Turabian StyleMartínez-Lorente, Sara Esperanza, José Manuel Martí-Guillén, María Ángeles Pedreño, Lorena Almagro, and Ana Belén Sabater-Jara. 2024. "Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress" Antioxidants 13, no. 3: 318. https://doi.org/10.3390/antiox13030318