Effect of Diet and Oxidative Stress in the Pathogenesis of Lymphoproliferative Disorders
Abstract
:1. Introduction
1.1. Diet and Cancer
1.2. Oxidative Stress and Cancer
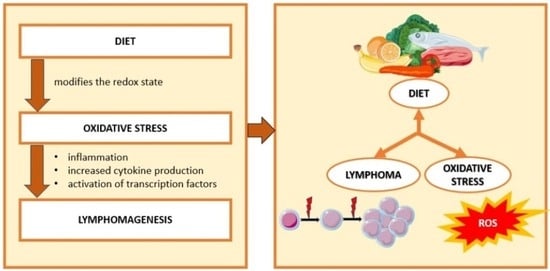
1.3. Correlation between Diet, Oxidative Stress, and Cancer
1.4. Oxidative Stress in Lymphoma
The Main Subtypes of Lymphoproliferative Disorders and Their Correlation with Oxidative Stress
2. Lymphoma, Diet, and Oxidative Stress
| Study Title | Object of the Study | Main Outcomes | Reference |
|---|---|---|---|
| Effect of β-carotene on catechol-induced genotoxicity in vitro: evidence of both enhanced and reduced DNA damage | In vitro Mouse lymphoma L5178Y cells | β-carotene reduces oxidative DNA damage for catechol concentrations lower than or equal to 0.75 mM, while it potentiates it for catechol concentrations greater than or equal to 1 mM. | [167] |
| Phytochemical profile and antioxidation activity of Anonna fruit and its effect on lymphoma cell proliferation | In vitro Burkitt lymphoma cell line Ramos-1, CRL-1596 | The methanol skin extracts had the highest phenol, flavonoid, tannin content, and antioxidant activity. Methanol extracts of the skin, pulp, and seeds had a modest effect, whereas chloroform extracts of the pulp and seeds had potent effects. | [179] |
| Isolation and characterization of anti-inflammatory and antiproliferative compound for B-cell non-Hodgkin lymphoma from Nyctanthes arbor-tristis Linn. | In vitro Follicular lymphoma cell line DOHH2 and Burkitt lymphoma cell line Raji | Nyctanthesin A showed anti-inflammatory and antioxidant activity, and in lymphoma cell lines, it exhibited antiproliferative action, also acting on gene and protein expression. | [180] |
| Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting | In vivo OF1 mice | Mice kept on alternate-day fasting had a lower oxidative state and significantly reduced incidence of lymphoma, compared with mice fed ad libitum. | [181] |
| Dietary restriction attenuates the accelerated aging phenotype of Sod1-/- mice | In vivo Sod1-/- mice | Dietary restriction resulted in a 30% increase in the lifespan of Sod1-/- mice compared with controls, a significant reduction in oxidative damage, and a reduction in the incidence of lymphomas. | [182] |
| Genetic polymorphisms in nitric oxide synthase genes modify the relationship between vegetable and fruit intake and risk of non-Hodgkin lymphoma | In vivo Female NHL patients | The risk of lymphoma varies with dietary intake of fruits and vegetables in the presence of particular SNPs, such as NOS1, NOS2A, MPO, and SOD3, with more significant results for NOS1. | [45] |
| Cellular oxidative stress in pediatric leukemia and lymphoma patients undergoing treatment is associated with protein consumption | In vivo pediatric leukemia and lymphoma patients | Intracellular levels of superoxide, peroxide, and glutathione increased during the six months of chemotherapy treatment and were associated with the consumption of animal protein, plant protein, and total protein; glutathione was positively correlated with the intake of plant protein. | [183] |
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Riboli, E. Diet and cancer—The European Prospective Investigation into Cancer and Nutrition. Nat. Rev. Cancer 2004, 4, 206–215. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.J.; Colditz, G.A. Modifiable risk factors for cancer. Br. J. Cancer 2004, 90, 299–303. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Cancer 2010, 46, 2555–2562. [Google Scholar] [CrossRef]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297. [Google Scholar] [CrossRef]
- Colditz, G.A.; Atwood, K.A.; Emmons, K.; Monson, R.R.; Willett, W.C.; Trichopoulos, D.; Hunter, D.J. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control 2000, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer-World Health Organization. World Cancer Report: Cancer Research for Cancer Prevention; World Health Organization: Lyon, France, 2020.
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of High-dose Vitamin C Combined with Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Prakash, M.D.; Kuol, N.; Nurgali, K.; Stojanovska, L.; Apostolopoulos, V. Anti-Tumor Effects of Vitamin B2, B6 and B9 in Promonocytic Lymphoma Cells. Int. J. Mol. Sci. 2019, 20, 3763. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Clark, L.C.; Turnbull, B.W. An analysis of cancer prevention by selenium. Biofactors 2001, 14, 153–159. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2018. [Google Scholar]
- Gilsing, A.M.; Fransen, F.; de Kok, T.M.; Goldbohm, A.R.; Schouten, L.J.; de Bruïne, A.P.; van Engeland, M.; van den Brandt, P.A.; de Goeij, A.F.; Weijenberg, M.P. Dietary heme iron and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis 2013, 34, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 2006, 65, 278–290. [Google Scholar] [CrossRef]
- Fanale, D.; Maragliano, R.; Perez, A.; Russo, A. Effects of Dietary Restriction on Cancer Development and Progression. In Handbook of Famine, Starvation, and Nutrient Deprivation; Preedy, V., Patel, V., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Gillespie, Z.E.; Pickering, J.; Eskiw, C.H. Better Living through Chemistry: Caloric Restriction (CR) and CR Mimetics Alter Genome Function to Promote Increased Health and Lifespan. Front. Genet. 2016, 7, 142. [Google Scholar] [CrossRef]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef]
- Clinthorne, J.F.; Beli, E.; Duriancik, D.M.; Gardner, E.M. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J. Immunol. 2013, 190, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Semeraro, M.; Enot, D.P.; Chaba, K.; Poirier Colame, V.; Dartigues, P.; Perier, A.; Villa, I.; Rusakiewicz, S.; Gronnier, C.; et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget 2015, 6, 20840–20850. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Pol, J.; Kroemer, G. Fasting improves anticancer immunosurveillance via autophagy induction in malignant cells. Cell Cycle 2016, 15, 3327–3328. [Google Scholar] [CrossRef]
- Pistollato, F.; Forbes-Hernandez, T.Y.; Iglesias, R.C.; Ruiz, R.; Elexpuru Zabaleta, M.; Dominguez, I.; Cianciosi, D.; Quiles, J.L.; Giampieri, F.; Battino, M. Effects of caloric restriction on immunosurveillance, microbiota and cancer cell phenotype: Possible implications for cancer treatment. Semin. Cancer Biol. 2021, 73, 45–57. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Preiser, J.C. Oxidative stress. JPEN J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Lau, A.T.; Wang, Y.; Chiu, J.F. Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J. Cell Biochem. 2008, 104, 657–667. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Giordano, L.; Musolino, C.; Gangemi, S. Targeting Redox Regulation as a Therapeutic Opportunity against Acute Leukemia: Pro-Oxidant Strategy or Antioxidant Approach? Antioxidants 2022, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Park, A.M.; Kira, Y.; Imada, I.; Utsumi, K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr. Med. Chem. 2003, 10, 2495–2505. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase: An update. Blood 1999, 93, 1464–1476. [Google Scholar] [CrossRef]
- Tulard, A.; Hoffschir, F.; de Boisferon, F.H.; Luccioni, C.; Bravard, A. Persistent oxidative stress after ionizing radiation is involved in inherited radiosensitivity. Free Radic. Biol. Med. 2003, 35, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef]
- Corton, J.C.; Cunningham, M.L.; Hummer, B.T.; Lau, C.; Meek, B.; Peters, J.M.; Popp, J.A.; Rhomberg, L.; Seed, J.; Klaunig, J.E. Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit. Rev. Toxicol. 2014, 44, 1–49. [Google Scholar] [CrossRef]
- Del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L.; Ramirez-Tortosa, M.; Perez-Lopez, P.; Granados-Principal, S.; Battino, M.; Quiles, J.L. Long-term effects of systemic cancer treatment on DNA oxidative damage: The potential for targeted therapies. Cancer Lett. 2012, 327, 134–141. [Google Scholar] [CrossRef]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative stress and reactive oxygen species. Contrib. Nephrol. 2005, 149, 240–260. [Google Scholar] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 Pt 5, 1147–1150. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, W.; Chen, X.; Wang, S.; Qian, W. Oxidative stress response induced by chemotherapy in leukemia treatment. Mol. Clin. Oncol. 2018, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kilfoy, B.A.; Zheng, T.; Lan, Q.; Han, X.; Qin, Q.; Rothman, N.; Holford, T.; Zhang, Y. Genetic polymorphisms in nitric oxide synthase genes modify the relationship between vegetable and fruit intake and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1429–1438. [Google Scholar]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Dehennaut, V.; Loison, I.; Dubuissez, M.; Nassour, J.; Abbadie, C.; Leprince, D. DNA double-strand breaks lead to activation of hypermethylated in cancer 1 (HIC1) by SUMOylation to regulate DNA repair. J. Biol. Chem. 2013, 288, 10254–10264. [Google Scholar] [CrossRef]
- Olinski, R.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Roszkowski, K.; Jaruga, P. Oxidative DNA damage: Assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic. Biol. Med. 2002, 33, 192–200. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Hildeman, D.A.; Mitchell, T.; Teague, T.K.; Henson, P.; Day, B.J.; Kappler, J.; Marrack, P.C. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 1999, 10, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Sim, J.E.; Ridnour, L.A.; Galoforo, S.S.; Lee, Y.J. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann. N. Y. Acad. Sci. 2000, 899, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. PI-103 and Quercetin Attenuate PI3K-AKT Signaling Pathway in T-Cell Lymphoma Exposed to Hydrogen Peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med. 2004, 36, 718–744. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- De Marco, F.; Bucaj, E.; Foppoli, C.; Fiorini, A.; Blarzino, C.; Filipi, K.; Giorgi, A.; Schininà, M.E.; Di Domenico, F.; Coccia, R.; et al. Oxidative stress in HPV-driven viral carcinogenesis: Redox proteomics analysis of HPV-16 dysplastic and neoplastic tissues. PLoS ONE 2012, 7, e34366. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M. Oxidatively induced DNA damage: Mechanisms, repair and disease. Cancer Lett. 2012, 327, 26–47. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Jackson, A.L.; Loeb, L.A. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res. 2001, 477, 7–21. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.M.; Cahill, M.A.; Rupec, R.A.; Baeuerle, P.A.; Nordheim, A. Antioxidants as well as oxidants activate c-fos via Ras-dependent activation of extracellular-signal-regulated kinase 2 and Elk-1. Eur. J. Biochem. 1997, 244, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef]
- Baldwin, A.S., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef]
- Rath, P.C.; Aggarwal, B.B. Antiproliferative effects of IFN-alpha correlate with the downregulation of nuclear factor-kappa B in human Burkitt lymphoma Daudi cells. J. Interferon Cytokine Res. 2001, 21, 523–528. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic Regulation of Redox Balance in Cancer. Cancers 2019, 11, 955. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Lin, Z.; Trimmer, C.; Flomenberg, N.; Wang, C.; Pavlides, S.; Pestell, R.G.; Howell, A.; Sotgia, F.; Lisanti, M.P. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: Implications for PET imaging of human tumors. Cell Cycle 2011, 10, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, W.; Liu, J.; Zhang, Y.; Qin, H.; Wu, H.; Xue, B.; Lu, Y.; Shen, P. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid. Redox Signal. 2013, 19, 1337–1355. [Google Scholar] [CrossRef]
- Beyer, M.; Schultze, J.L. Regulatory T cells in cancer. Blood 2006, 108, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Dual effect of oxidative stress on leukemia cancer induction and treatment. J. Exp. Clin. Cancer Res. 2014, 33, 106. [Google Scholar] [CrossRef] [PubMed]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; Araújo Mdo, C.; Schetinger, M.R.; Morsch, V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Robert, C.; Gough, S.M.; Rassool, F.V.; Aplan, P.D. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leuk. Res. 2014, 38, 95–102. [Google Scholar] [CrossRef]
- Pawlowska, E.; Blasiak, J. DNA Repair—A Double-Edged Sword in the Genomic Stability of Cancer Cells—The Case of Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2015, 16, 27535–27549. [Google Scholar] [CrossRef]
- Testa, U.; Labbaye, C.; Castelli, G.; Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 2016, 44, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bassig, B.A.; Seow, W.J.; Hu, W.; Purdue, M.P.; Huang, W.Y.; Liu, C.S.; Cheng, W.L.; Männistö, S.; Vermeulen, R.; et al. Mitochondrial DNA copy number and chronic lymphocytic leukemia/small lymphocytic lymphoma risk in two prospective studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 148–153. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Casciaro, M.; Musolino, C.; Gangemi, S. Synergic Crosstalk between Inflammation, Oxidative Stress, and Genomic Alterations in BCR-ABL-Negative Myeloproliferative Neoplasm. Antioxidants 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.L.; Yao, S.; Goldman, B.H.; Lee, K.; Spier, C.M.; LeBlanc, M.L.; Rimsza, L.M.; Cerhan, J.R.; Habermann, T.M.; Link, B.K.; et al. Genetic polymorphisms in oxidative stress-related genes are associated with outcomes following treatment for aggressive B-cell non-Hodgkin lymphoma. Am. J. Hematol. 2014, 89, 639–645. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Polito, F.; Oteri, R.; Alibrandi, A.; Allegra, A.G.; Oteri, G.; Di Giorgio, R.M.; Musolino, C.; Aguennouz, M. SIRT2 and SIRT3 expression correlates with redox imbalance and advanced clinical stage in patients with multiple myeloma. Clin. Biochem. 2021, 93, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Saija, A.; Alonci, A.; Russo, S.; Spatari, G.; Penna, G.; Gerace, D.; Cristani, M.; David, A.; et al. Changes in advanced oxidation protein products, advanced glycation end products, and s-nitrosylated proteins, in patients affected by polycythemia vera and essential thrombocythemia. Clin. Biochem. 2012, 45, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pace, E.; Tartarisco, G.; Innao, V.; DISalvo, E.; Allegra, A.G.; Ferraro, M.; Musolino, C.; Gangemi, S. Changes in Serum Interleukin-8 and sRAGE Levels in Multiple Myeloma Patients. Anticancer Res. 2020, 40, 1443–1449. [Google Scholar] [CrossRef]
- Allegra, A.; Musolino, C.; Pace, E.; Innao, V.; Di Salvo, E.; Ferraro, M.; Casciaro, M.; Spatari, G.; Tartarisco, G.; Allegra, A.G.; et al. Evaluation of the AGE/sRAGE Axis in Patients with Multiple Myeloma. Antioxidants 2019, 8, 55. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papalou, O.; Kandaraki, E.A.; Kassi, G. MECHANISMS IN ENDOCRINOLOGY: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur. J. Endocrinol. 2017, 176, R79–R99. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007, 27, 817–868. [Google Scholar] [CrossRef]
- Mohanty, P.; Hamouda, W.; Garg, R.; Aljada, A.; Ghanim, H.; Dandona, P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J. Clin. Endocrinol. Metab. 2000, 85, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Ghanim, H.; Hamouda, W.; Aljada, A.; Garg, R.; Dandona, P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002, 75, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Shahedi, K.; Pandol, S.J.; Hu, R. Oxidative stress and alcoholic pancreatitis. J. Gastroenterol. Hepatol. Res. 2013, 2, 335–342. [Google Scholar]
- Kandi, S.; Deshpande, N.; Pinnelli, V.B.K.; Devaki, R.; Rao, P.; Ramana, K. Alcoholism and its role in the development of oxidative stress and DNA damage: An Insight. Am. J. Med. Sci. Med. 2014, 2, 64–66. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Bailey, S.M. Ethanol consumption and liver mitochondria function. Biol. Signals Recept. 2001, 10, 271–282. [Google Scholar] [CrossRef]
- Gregersen, S.; Samocha-Bonet, D.; Heilbronn, L.K.; Campbell, L.V. Inflammatory and oxidative stress responses to high-carbohydrate and high-fat meals in healthy humans. J. Nutr. Metab. 2012, 2012, 238056. [Google Scholar] [CrossRef]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Hu, Y.; Block, G.; Norkus, E.P.; Morrow, J.D.; Dietrich, M.; Hudes, M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am. J. Clin. Nutr. 2006, 84, 70–76; quiz 266-7. [Google Scholar] [CrossRef]
- Amador-Licona, N.; Díaz-Murillo, T.A.; Gabriel-Ortiz, G.; Pacheco-Moises, F.P.; Pereyra-Nobara, T.A.; Guízar-Mendoza, J.M.; Barbosa-Sabanero, G.; Orozco-Aviña, G.; Moreno-Martínez, S.C.; Luna-Montalbán, R.; et al. Omega 3 Fatty Acids Supplementation and Oxidative Stress in HIV-Seropositive Patients. A Clinical Trial. PLoS ONE 2016, 11, e0151637. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef]
- Nance, S.A.; A’ja, V.D.; Gwathmey, T.M.; Hairston, K.G. Soluble dietary fiber in obesity-associated inflammation and oxidative stress in African American women. FASEB J. 2017, 31, 434.2. [Google Scholar]
- Sánchez, D.; Quiñones, M.; Moulay, L.; Muguerza, B.; Miguel, M.; Aleixandre, A. Soluble fiber-enriched diets improve inflammation and oxidative stress biomarkers in Zucker fatty rats. Pharmacol. Res. 2011, 64, 31–35. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Lam, Y.Y.; Mano, M.; Wittert, G.A.; Bird, A.R. Cereal based diets modulate some markers of oxidative stress and inflammation in lean and obese Zucker rats. Nutr. Metab. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Costa Marques, T.H.; Santos De Melo, C.H.; Fonseca De Carvalho, R.B.; Costa, L.M.; De Souza, A.A.; David, J.M.; De Lima David, J.P.; De Freitas, R.M. Phytochemical profile and qualification of biological activity of an isolated fraction of Bellis perennis. Biol. Res. 2013, 46, 231–238. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Oliveira, A.A.; Loureiro, A.P.; Gattás, G.J.; Fisberg, R.M.; Marchioni, D.M. Arginine intake is associated with oxidative stress in a general population. Nutrition 2017, 33, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Meat and cancer. Meat Sci. 2010, 84, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Elsner, A.; Proll, J.; Thielecke, F.; Metges, C.C. Long-term high protein intake does not increase oxidative stress in rats. J. Nutr. 2000, 130, 2889–2896. [Google Scholar] [CrossRef]
- Aljada, A.; Mohanty, P.; Ghanim, H.; Abdo, T.; Tripathy, D.; Chaudhuri, A.; Dandona, P. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: Evidence for a proinflammatory effect. Am. J. Clin. Nutr. 2004, 79, 682–690. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shaik, S.; Negi, B.S.; Rajguru, J.P.; Patil, P.B.; Parihar, A.S.; Sharma, U. Non-Hodgkin’s lymphoma: A review. J. Fam. Med. Prim. Care 2020, 9, 1834–1840. [Google Scholar]
- Matasar, M.J.; Zelenetz, A.D. Overview of lymphoma diagnosis and management. Radiol. Clin. N. Am. 2008, 46, 175–198. [Google Scholar] [CrossRef]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care 2016, 43, 661–675. [Google Scholar] [CrossRef]
- Ansell, S.M. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin. Proc. 2015, 90, 1152–1163. [Google Scholar] [CrossRef]
- Connors, J.M. Non-Hodgkin lymphoma: The clinician’s perspective—A view from the receiving end. Mod. Pathol. 2013, 26 (Suppl. S1), S111–S118. [Google Scholar] [CrossRef]
- Morton, L.M.; Slager, S.L.; Cerhan, J.R.; Wang, S.S.; Vajdic, C.M.; Skibola, C.F.; Bracci, P.M.; de Sanjosé, S.; Smedby, K.E.; Chiu, B.C.; et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 130–144. [Google Scholar] [CrossRef]
- Mancuso, S.; Carlisi, M.; Santoro, M.; Napolitano, M.; Raso, S.; Siragusa, S. Immunosenescence and lymphomagenesis. Immun. Ageing 2018, 15, 22. [Google Scholar] [CrossRef]
- Noy, A. HIV Lymphoma and Burkitts Lymphoma. Cancer J. 2020, 26, 260–268. [Google Scholar] [CrossRef]
- Vockerodt, M.; Yap, L.F.; Shannon-Lowe, C.; Curley, H.; Wei, W.; Vrzalikova, K.; Murray, P.G. The Epstein-Barr virus and the pathogenesis of lymphoma. J. Pathol. 2015, 235, 312–322. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Shabani, M.; Tabary, M.; Rayzan, E.; Rezaei, N. Lymphoma in the setting of autoimmune diseases: A review of association and mechanisms. Crit. Rev. Oncol. Hematol. 2020, 150, 102945. [Google Scholar] [CrossRef]
- Corbeanu, R.I.; Găman, A.M. The involvement of oxidative stress in non-Hodgkin’s lymphomas; a review of the literature. J. Mind Med. Sci. 2022, 9, 2. [Google Scholar] [CrossRef]
- Candi, E.; Agostini, M.; Melino, G.; Bernassola, F. How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: Regulators and effectors. Hum. Mutat. 2014, 35, 702–714. [Google Scholar] [CrossRef]
- Voropaeva, E.N.; Pospelova, T.I.; Voevoda, M.I.; Maksimov, V.N.; Orlov, Y.L.; Seregina, O.B. Clinical aspects of TP53 gene inactivation in diffuse large B-cell lymphoma. BMC Med. Genom. 2019, 12 (Suppl. S2), 35. [Google Scholar] [CrossRef]
- Ma, E.S. Recurrent Cytogenetic Abnormalities in Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. Methods Mol. Biol. 2017, 1541, 279–293. [Google Scholar]
- Pasqualucci, L.; Dalla-Favera, R. Genetics of diffuse large B-cell lymphoma. Blood 2018, 131, 2307–2319. [Google Scholar] [CrossRef]
- López, C.; Kleinheinz, K.; Aukema, S.M.; Rohde, M.; Bernhart, S.H.; Hübschmann, D.; Wagener, R.; Toprak, U.H.; Raimondi, F.; Kreuz, M.; et al. Genomic and transcriptomic changes complement each other in the pathogenesis of sporadic Burkitt lymphoma. Nat. Commun. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Kourentzi, K.; Crum, M.; Patil, U.; Prebisch, A.; Chavan, D.; Vu, B.; Zeng, Z.; Litvinov, D.; Zu, Y.; Willson, R.C. Recombinant expression, characterization, and quantification in human cancer cell lines of the Anaplastic Large-Cell Lymphoma-characteristic NPM-ALK fusion protein. Sci. Rep. 2020, 10, 5078. [Google Scholar] [CrossRef]
- Jain, A.G.; Chang, C.C.; Ahmad, S.; Mori, S. Leukemic Non-nodal Mantle Cell Lymphoma: Diagnosis and Treatment. Curr. Treat. Options Oncol. 2019, 20, 85. [Google Scholar] [CrossRef]
- Yin, C.C.; Luthra, R. Molecular detection of t(11;14)(q13;q32) in mantle cell lymphoma. Methods Mol. Biol. 2013, 999, 211–216. [Google Scholar]
- Sousa-Pimenta, M.; Estevinho, M.M.; Sousa Dias, M.; Martins, Â.; Estevinho, L.M. Oxidative Stress and Inflammation in B-Cell Lymphomas. Antioxidants 2023, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Janz, S.; Potter, M.; Rabkin, C.S. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes Chromosomes Cancer 2003, 36, 211–223. [Google Scholar] [CrossRef]
- Parascandolo, A.; Laukkanen, M.O. Carcinogenesis and Reactive Oxygen Species Signaling: Interaction of the NADPH Oxidase NOX1-5 and Superoxide Dismutase 1-3 Signal Transduction Pathways. Antioxid. Redox Signal. 2019, 30, 443–486. [Google Scholar] [CrossRef]
- Lang, J.Y.; Ma, K.; Guo, J.X.; Sun, H. Oxidative stress induces B lymphocyte DNA damage and apoptosis by upregulating p66shc. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1051–1060. [Google Scholar]
- Zhou, F.; Shen, Q.; Claret, F.X. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J. Leukoc. Biol. 2013, 94, 423–429. [Google Scholar] [CrossRef]
- Wang, L.; Howell, M.E.A.; Sparks-Wallace, A.; Hawkins, C.; Nicksic, C.A.; Kohne, C.; Hall, K.H.; Moorman, J.P.; Yao, Z.Q. p62-mediated Selective autophagy endows virus-transformed cells with insusceptibility to DNA damage under oxidative stress. PLoS Pathog. 2019, 15, e1007541. [Google Scholar] [CrossRef]
- Wang, S.S.; Davis, S.; Cerhan, J.R.; Hartge, P.; Severson, R.K.; Cozen, W.; Lan, Q.; Welch, R.; Chanock, S.J.; Rothman, N. Polymorphisms in oxidative stress genes and risk for non-Hodgkin lymphoma. Carcinogenesis 2006, 27, 1828–1834. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.T.; Wang, L.W.; Xu, Y.C.; Wang, X.R. A novel genetic polymorphism of inducible nitric oxide synthase is associated with an increased risk of gastric cancer. World J. Gastroenterol. 2004, 10, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Zheng, T.; Shen, M.; Zhang, Y.; Wang, S.S.; Zahm, S.H.; Holford, T.R.; Leaderer, B.; Boyle, P.; Chanock, S. Genetic polymorphisms in the oxidative stress pathway and susceptibility to non-Hodgkin lymphoma. Hum. Genet. 2007, 121, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, T.J.; Skibola, C.F.; Smith, A.G.; Forrest, M.S.; Adamson, P.J. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin’s lymphoma. Haematologica 2006, 91, 1222–1227. [Google Scholar] [PubMed]
- Farawela, H.; Khorshied, M.; Shaheen, I.; Gouda, H.; Nasef, A.; Abulata, N.; Mahmoud, H.A.; Zawam, H.M.; Mousa, S.M. The association between hepatitis C virus infection, genetic polymorphisms of oxidative stress genes and B-cell non-Hodgkin’s lymphoma risk in Egypt. Infect. Genet. Evol. 2012, 12, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef]
- Peroja, P.; Haapasaari, K.M.; Mannisto, S.; Miinalainen, I.; Koivunen, P.; Leppä, S.; Karjalainen-Lindsberg, M.L.; Kuusisto, M.E.; Turpeenniemi-Hujanen, T.; Kuittinen, O.; et al. Total peroxiredoxin expression is associated with survival in patients with follicular lymphoma. Virchows Arch. 2016, 468, 623–630. [Google Scholar] [CrossRef]
- Gaman, M.A.; Epingeac, M.E.; Gaman, A.M. Evaluation of oxidative stress and high-density lipoprotein cholesterol levels in diffuse large B-cell lymphoma. Rev. Chim. 2019, 70, 977–980. [Google Scholar] [CrossRef]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef]
- Ghareeb, H.; Metanis, N. The Thioredoxin System: A Promising Target for Cancer Drug Development. Chemistry 2020, 26, 10175–10184. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Jia, J.J.; Geng, W.S.; Wang, Z.Q.; Chen, L.; Zeng, X.S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Woods, K.; Di Trapani, G.; Tonissen, K.F. Investigating the Thioredoxin and Glutathione Systems’ Response in Lymphoma Cells after Treatment with [Au(d2pype)2]CL. Antioxidants 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Collado, R.; Ivars, D.; Oliver, I.; Tormos, C.; Egea, M.; Miguel, A.; Sáez, G.T.; Carbonell, F. Increased oxidative damage associated with unfavorable cytogenetic subgroups in chronic lymphocytic leukemia. Biomed. Res. Int. 2014, 2014, 686392. [Google Scholar] [CrossRef]
- D’Arena, G.; Seneca, E.; Migliaccio, I.; De Feo, V.; Giudice, A.; La Rocca, F.; Capunzo, M.; Calapai, G.; Festa, A.; Caraglia, M.; et al. Oxidative stress in chronic lymphocytic leukemia: Still a matter of debate. Leuk. Lymphoma 2019, 60, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Jitschin, R.; Hofmann, A.D.; Bruns, H.; Giessl, A.; Bricks, J.; Berger, J.; Saul, D.; Eckart, M.J.; Mackensen, A.; Mougiakakos, D. Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia. Blood 2014, 123, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; Papendrecht-van der Voort, E.A.; Leow, A.; Nivine Levarht, E.W.; Breedveld, F.C.; Verweij, C.L. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol. Cell. Biol. 2002, 22, 400–411. [Google Scholar] [CrossRef]
- Nindl, G.; Peterson, N.R.; Hughes, E.F.; Waite, L.R.; Johnson, M.T. Effect of hydrogen peroxide on proliferation, apoptosis and interleukin-2 production of Jurkat T cells. Biomed. Sci. Instrum. 2004, 40, 123–128. [Google Scholar]
- Cemerski, S.; Cantagrel, A.; Van Meerwijk, J.P.; Romagnoli, P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J. Biol. Chem. 2002, 277, 19585–19593. [Google Scholar] [CrossRef]
- Rider, D.A.; Sinclair, A.J.; Young, S.P. Oxidative inactivation of CD45 protein tyrosine phosphatase may contribute to T lymphocyte dysfunction in the elderly. Mech. Ageing Dev. 2003, 124, 191–198. [Google Scholar] [CrossRef]
- Kumar, S.; Dhamija, B.; Attrish, D.; Sawant, V.; Sengar, M.; Thorat, J.; Shet, T.; Jain, H.; Purwar, R. Genetic alterations and oxidative stress in T cell lymphomas. Pharmacol. Ther. 2022, 236, 108109. [Google Scholar] [CrossRef] [PubMed]
- Bur, H.; Haapasaari, K.M.; Turpeenniemi-Hujanen, T.; Kuittinen, O.; Auvinen, P.; Marin, K.; Koivunen, P.; Sormunen, R.; Soini, Y.; Karihtala, P. Oxidative stress markers and mitochondrial antioxidant enzyme expression are increased in aggressive Hodgkin lymphomas. Histopathology 2014, 65, 319–327. [Google Scholar]
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid peroxidation and protein oxidation in patients affected by Hodgkin’s lymphoma. Mediat. Inflamm. 2004, 13, 381–383. [Google Scholar] [CrossRef]
- Åsgård, R.; Hellman, B. Effect of β-carotene on catechol-induced genotoxicity in vitro: Evidence of both enhanced and reduced DNA damage. Free Radic. Res. 2013, 47, 692–698. [Google Scholar] [CrossRef]
- Di Mascio, P.; Murphy, M.E.; Sies, H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991, 53, 194S–200S. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of beta-carotene as an antioxidant against lipid peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]
- Krinsky, N.I. Carotenoids as antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef]
- Ringer, T.V.; DeLoof, M.J.; Winterrowd, G.E.; Francom, S.F.; Gaylor, S.K.; Ryan, J.A.; Sanders, M.E.; Hughes, G.S. Beta-carotene’s effects on serum lipoproteins and immunologic indices in humans. Am. J. Clin. Nutr. 1991, 53, 688–694. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Astley, S.B.; Hughes, D.A.; Wright, A.J.; Elliott, R.M.; Southon, S. DNA damage and susceptibility to oxidative damage in lymphocytes: Effects of carotenoids in vitro and in vivo. Br. J. Nutr. 2004, 91, 53–61. [Google Scholar] [CrossRef]
- van Helden, Y.G.; Keijer, J.; Knaapen, A.M.; Heil, S.G.; Briedé, J.J.; van Schooten, F.J.; Godschalk, R.W. Beta-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic. Biol. Med. 2009, 46, 299–304. [Google Scholar] [CrossRef]
- Siems, W.; Sommerburg, O.; Schild, L.; Augustin, W.; Langhans, C.D.; Wiswedel, I. Beta-carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002, 16, 1289–1291. [Google Scholar] [CrossRef]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Beta-carotene breakdown products enhance genotoxic effects of oxidative stress in primary rat hepatocytes. Carcinogenesis 2006, 27, 1128–1133. [Google Scholar] [CrossRef]
- Alija, A.J.; Bresgen, N.; Bojaxhi, E.; Vogl, C.; Siems, W.; Eckl, P.M. Cytotoxicity of β-carotene cleavage products and its prevention by antioxidants. Acta Biochim. Pol. 2010, 57, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.L.; Wang, H.M.; Chen, P.Y.; Wu, T.C. Interactions of beta-carotene and flavonoids on the secretion of pro-inflammatory mediators in an in vitro system. Chem. Biol. Interact. 2009, 179, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaya, H.M.; Li, H.; Beg, O.U.; Hamama, A.A.; Witiak, S.M.; Kaseloo, P.; Siddiqui, R.A. Phytochemical profile and antioxidation activity of annona fruit and its effect on lymphoma cell proliferation. Food Sci. Nutr. 2019, 8, 58–68. [Google Scholar] [CrossRef]
- Sana, T.; Qayyum, S.; Jabeen, A.; Siddiqui, B.S.; Begum, S.; Siddiqui, R.A.; Hadda, T.B. Isolation and characterization of anti-inflammatory and anti-proliferative compound, for B-cell Non-Hodgkin lymphoma, from Nyctanthes arbor-tristis Linn. J. Ethnopharmacol. 2022, 293, 115267. [Google Scholar]
- Descamps, O.; Riondel, J.; Ducros, V.; Roussel, A.M. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: Effect of alternate-day fasting. Mech. Ageing Dev. 2005, 126, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ikeno, Y.; Bokov, A.; Gelfond, J.; Jaramillo, C.; Zhang, H.M.; Liu, Y.; Qi, W.; Hubbard, G.; Richardson, A.; et al. Dietary restriction attenuates the accelerated aging phenotype of Sod1(-/-) mice. Free Radic. Biol. Med. 2013, 60, 300–306. [Google Scholar] [CrossRef]
- Raber, M.; Wu, J.; Donnella, H.; Knouse, P.; Pise, M.; Munsell, M.; Liu, D.; Chandra, J. Cellular Oxidative Stress in Pediatric Leukemia and Lymphoma Patients Undergoing Treatment Is Associated with Protein Consumption. Nutrients 2019, 12, 75. [Google Scholar] [CrossRef]
- American Chemical Society. Cancer Facts & Figures 2023; American Chemical Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Hodgson, D.C. Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: Optimizing treatment for individual patients. Clin. Adv. Hematol. Oncol. 2015, 13, 103–112. [Google Scholar] [PubMed]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Cicero, N.; Gangemi, S.; Allegra, A. Natural products and oxidative stress: Potential agents against multiple myeloma. Nat. Prod. Res. 2023, 37, 687–690. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Termini, D.; Albano, A.; Tsiani, E. Anti-Cancer Properties of Theaflavins. Molecules 2021, 26, 987. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Spagnolo, E.V.; Musolino, C.; Gangemi, S. Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies. Int. J. Mol. Sci. 2020, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Genovese, C.; Cicero, N.; Gangemi, S.; Allegra, A. The Anti-Cancer Effect of Cinnamon Aqueous Extract: A Focus on Hematological Malignancies. Life 2023, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Johnson, J.J. Rosemary (Salvia rosmarinus): Health-promoting benefits and food preservative properties. Int. J. Nutr. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Doello, K.; Ortiz, R.; Alvarez, P.J.; Melguizo, C.; Cabeza, L.; Prados, J. Latest in Vitro and in Vivo Assay, Clinical Trials and Patents in Cancer Treatment using Curcumin: A Literature Review. Nutr. Cancer 2018, 70, 569–578. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investg. 2017, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Speciale, A.; Molonia, M.S.; Guglielmo, L.; Musolino, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F. Curcumin ameliorates the in vitro efficacy of carfilzomib in human multiple myeloma U266 cells targeting p53 and NF-κB pathways. Toxicol. In Vitro 2018, 47, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Günal-Köroğlu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.M.; Al-Omari, B.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023, 161, 114428. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Mulder, T.P.; van Platerink, C.J.; Wijnand Schuyl, P.J.; van Amelsvoort, J.M. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Tirona, R.G.; Bailey, D.G. Herbal product-drug interactions mediated by induction. Br. J. Clin. Pharmacol. 2006, 61, 677–681. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancemi, G.; Cicero, N.; Allegra, A.; Gangemi, S. Effect of Diet and Oxidative Stress in the Pathogenesis of Lymphoproliferative Disorders. Antioxidants 2023, 12, 1674. https://doi.org/10.3390/antiox12091674
Cancemi G, Cicero N, Allegra A, Gangemi S. Effect of Diet and Oxidative Stress in the Pathogenesis of Lymphoproliferative Disorders. Antioxidants. 2023; 12(9):1674. https://doi.org/10.3390/antiox12091674
Chicago/Turabian StyleCancemi, Gabriella, Nicola Cicero, Alessandro Allegra, and Sebastiano Gangemi. 2023. "Effect of Diet and Oxidative Stress in the Pathogenesis of Lymphoproliferative Disorders" Antioxidants 12, no. 9: 1674. https://doi.org/10.3390/antiox12091674