Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Seed Proanthocyanidin Extract (GSPE)
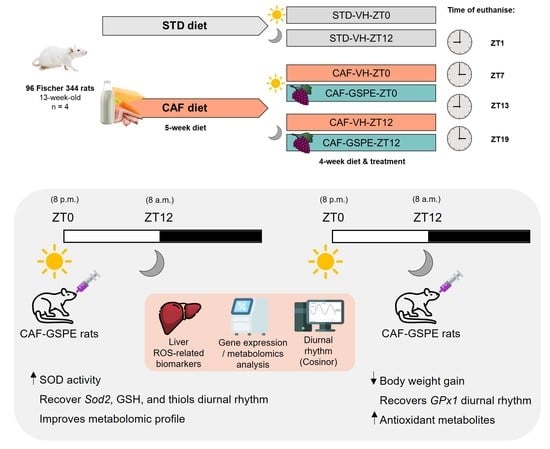
2.2. Animals and Experimental Procedure
2.3. Dosage Information/Dosage Regimen
2.4. Hepatic RNA Extraction
2.5. cDNA Synthesis and Gene-Expression Analysis
2.6. Serum Biochemical Analysis
2.7. Determination of AOX Activities and Lipid and Protein Oxidation in Liver Tissue
2.8. Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. GSPE Administration at ZT12 Reduces Body Weight Gain in CAF-Fed Animals and Partially Ameliorates Serum Parameters
3.2. GSPE Moderately Mitigates the Alterations in the ROS Detoxification System Originated by CAF in the Liver
3.3. The ROS Detoxification System in the Liver Is Altered Due to the CAF
3.4. GSPE Treatment at Both ZT0 and ZT12 Partially Ameliorate Hepatic AOX Metabolic Profiles Altered Due to the CAF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanford, A.B.A.; da Cunha, L.S.; Machado, C.B.; de Pinho Pessoa, F.M.C.; Silva, A.N.d.S.; Ribeiro, R.M.; Moreira, F.C.; de Moraes Filho, M.O.; de Moraes, M.E.A.; de Souza, L.E.B.; et al. Circadian Rhythm Dysregulation and Leukemia Development: The Role of Clock Genes as Promising Biomarkers. Int. J. Mol. Sci. 2022, 23, 8212. [Google Scholar] [CrossRef]
- Sato, T.; Sassone-Corsi, P. Nutrition, Metabolism, and Epigenetics: Pathways of Circadian Reprogramming. EMBO Rep. 2022, 23, e52412. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian Rhythm and Disease: Relationship, New Insights, and Future Perspectives. J. Cell Physiol. 2022, 237, 3239–3256. [Google Scholar] [CrossRef]
- Albrecht, U. The Circadian Clock, Metabolism and Obesity. Obes. Rev. 2017, 18, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016, 7, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, J.; Yao, K.; Su, W.; Tan, B.; Wu, X.; Huang, X.; Li, T.; Yin, Y.; Tosini, G.; et al. Circadian Rhythms and Obesity: Timekeeping Governs Lipid Metabolism. J. Pineal Res. 2020, 69, 12682. [Google Scholar] [CrossRef]
- Salgado-Delgado, R.C.; Saderi, N.; Basualdo, M.d.C.; Guerrero-Vargas, N.N.; Escobar, C.; Buijs, R.M. Shift Work or Food Intake during the Rest Phase Promotes Metabolic Disruption and Desynchrony of Liver Genes in Male Rats. PLoS ONE 2013, 8, e60052. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Laermans, J.; Depoortere, I. Chronobesity: Role of the Circadian System in the Obesity Epidemic. Obes. Rev. 2016, 17, 108–125. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and AOXs: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Vykhovanets, O.; Kondratova, A.A.; Antoch, M.P. AOX N-Acetyl-L-Cysteine Ameliorates Symptoms of Premature Aging Associated with the Deficiency of the Circadian Protein BMAL1. Aging 2009, 1, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived AOXs and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. AOXs 2021, 10, 708. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. AOX Dietary Approach in Treatment of Fatty Liver: New Insights and Updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, M.; Pikala, M.; Grygorczuk, O.; Waśkiewicz, A.; Stepaniak, U.; Pająk, A.; Kozakiewicz, K.; Nadrowski, P.; Zdrojewski, T.; Puch-Walczak, A.; et al. Dietary AOXs, Quality of Nutrition and Cardiovascular Characteristics among Omnivores, Flexitarians and Vegetarians in Poland—The Results of Multicenter National Representative Survey WOBASZ. AOXs 2023, 12, 222. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Cannataro, R.; Fazio, A.; Torre, C.L.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to MicroRNA Modulation. AOXs 2021, 10, 328. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely Administered Grape-Seed Proanthocyanidin Extract Acts as a Satiating Agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Montagut, G.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pinent, M.; Ardévol, A. Effects of a Grapeseed Procyanidin Extract (GSPE) on Insulin Resistance. J. Nutr. Biochem. 2010, 21, 961–967. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic Administration of Grape-Seed Polyphenols Attenuates the Development of Hypertension and Improves Other Cardiometabolic Risk Factors Associated with the Metabolic Syndrome in Cafeteria Diet-Fed Rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Puiggròs, F.; Llópiz, N.; Ardévol, A.; Bladé, C.; Arola, L.; Salvadó, M.J. Grape Seed Procyanidins Prevent Oxidative Injury by Modulating the Expression of AOX Enzyme Systems. J. Agric. Food Chem. 2005, 53, 6080–6086. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yun, P.; Hu, Y.; Yang, J.; Khadka, R.B.; Peng, X. Effects of Grape Seed Proanthocyanidin Extract on Obesity. Obes. Facts 2020, 13, 279–291. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Modulate Melatonin Levels in Plasma and the Expression Pattern of Clock Genes in the Hypothalamus of Rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Dietary Proanthocyanidins Modulate BMAL1 Acetylation, Nampt Expression and NAD Levels in Rat Liver. Sci. Rep. 2015, 5, srep10954. [Google Scholar] [CrossRef] [Green Version]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol Opposite Effects on Rat Tissue Lipoperoxidation: Pro-Oxidant during Day-Time and AOX at Night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Mulero, M.; Muguerza, B.; Suárez, M.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Microbiota Influences the Photoperiod Effects on Proanthocyanidins Bioavailability in Diet-Induced Obese Rats. Mol. Nutr. Food Res. 2023, 67, 2200600. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Muguerza, B.; Aragonès, G.; Suárez, M.; Torres-Fuentes, C.; Arola-Arnal, A. The Effects of Grape Seed Proanthocyanidins in Cafeteria Diet-Induced Obese Fischer 344 Rats Are Influenced by Faecal Microbiota in a Photoperiod Dependent Manner. Food Funct. 2022, 13, 8363–8374. [Google Scholar] [CrossRef]
- Zeeni, N.; Dagher-Hamalian, C.; Dimassi, H.; Faour, W.H. Cafeteria Diet-Fed Mice Is a Pertinent Model of Obesity-Induced Organ Damage: A Potential Role of Inflammation. Inflamm. Res. 2015, 64, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Boost Hepatic NAD+ Metabolism and SIRT1 Expression and Activity in a Dose-Dependent Manner in Healthy Rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Marklund, S. Spectrophotometric Study of Spontaneous Disproportionation of Superoxide Anion Radical and Sensitive Direct Assay for Superoxide Dismutase. J. Biol. Chem. 1976, 251, 7504–7507. [Google Scholar] [CrossRef]
- Beers, R.; Sizer, I. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzym. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.-L. Measurement of Plasma Sulfhydryl and Carbonyl Groups as a Possible Indicator of Protein Oxidation. In Analysis of Free Radicals in Biological Systems; Birkhäuser: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Nguyen Khoa, T.; Capeillère-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.M.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B. Advanced Oxidation Protein Products as Novel Mediators of Inflammation and Monocyte Activation in Chronic Renal Failure. J. Immunol. 1998, 161, 2524–2532. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-Based Inference of Biological Patterns, Functions and Pathways from Metabolomic Data Using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Moškon, M. CosinorPy: A Python Package for Cosinor-Based Rhythmometry. BMC Bioinform. 2020, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Moliner, C.; Núñez, S.; Cásedas, G.; Valero, M.S.; Dias, M.I.; Barros, L.; López, V.; Gómez-Rincón, C. Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and AOX Effects in Caenorhabditis Elegans. AOXs 2023, 12, 720. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, H.; Guo, H.; Li, Z.; Li, D.; Xie, Z. Tea Polyphenol EGCG Ameliorates Obesity-Related Complications by Regulating Lipidomic Pathway in Leptin Receptor Knockout Rats. J. Nutr. Biochem. 2023, 118, 109349. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Sánchez-Castillo, D.R.; Cariño-Cervantes, Y.Y.; Torre-Villalvazo, I.; Tovar-Palacio, C.; Vásquez-Reyes, S.; Furuzawa-Carballeda, J.; Acevedo-Carabantes, J.A.; Camacho-Corona, M.d.R.; Guzmán-Mar, J.L.; et al. Vachellia Farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2023, 24, 7984. [Google Scholar] [CrossRef]
- Maturana, G.; Segovia, J.; Olea-Azar, C.; Uribe-Oporto, E.; Espinosa, A.; Zúñiga-López, M.C. Evaluation of the Effects of Chia (Salvia hispanica L.) Leaves Ethanolic Extracts Supplementation on Biochemical and Hepatic Markers on Diet-Induced Obese Mice. AOXs 2023, 12, 1108. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural AOXs, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- González-Garrido, J.A.; García-Sánchez, J.R.; López-Victorio, C.J.; Escobar-Ramírez, A.; Olivares-Corichi, I.M. Cocoa: A Functional Food That Decreases Insulin Resistance and Oxidative Damage in Young Adults with Class II Obesity. Nutr. Res. Pract. 2023, 17, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Khan, A.S.; Khan, N.A.; Hichami, A. AOX and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Taïlé, J.; Bringart, M.; Planesse, C.; Patché, J.; Rondeau, P.; Veeren, B.; Clerc, P.; Gauvin-Bialecki, A.; Bourane, S.; Meilhac, O.; et al. AOX Polyphenols of Antirhea Borbonica Medicinal Plant and Caffeic Acid Reduce Cerebrovascular, Inflammatory and Metabolic Disorders Aggravated by High-Fat Diet-Induced Obesity in a Mouse Model of Stroke. AOXs 2022, 11, 858. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK Regulates the Circadian Clock by Cryptochrome Phosphorylation and Degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sani, M.; Sebai, H.; Ghanem-Boughanmi, N.; Boughattas, N.A.; Ben-Attia, M. Circadian (about 24-Hour) Variation in Malondialdehyde Content and Catalase Activity of Mouse Erythrocytes. Redox Rep. 2015, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Feeney, K.A. Circadian Redox and Metabolic Oscillations in Mammalian Systems. Antioxid. Redox Signal 2014, 20, 2966–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, M.K.; Mohandas, S.; Ramkumar, K.M. Dysfunctions, Molecular Mechanisms, and Therapeutic Strategies of Pancreatic β-Cells in Diabetes. Apoptosis 2023, 28, 958–976. [Google Scholar] [CrossRef]
- Cao, J.; Yu, X.; Deng, Z.; Pan, Y.; Zhang, B.; Tsao, R.; Li, H. Chemical Compositions, Antiobesity, and AOX Effects of Proanthocyanidins from Lotus Seed Epicarp and Lotus Seed Pot. J. Agric. Food Chem. 2018, 66, 13492–13502. [Google Scholar] [CrossRef]
- Fogacci, F.; Borghi, C.; Rizzoli, E.; Giovannini, M.; Bove, M.; D’addato, S.; Cicero, A.F.G. Effect of Dietary Supplementation with Eufortyn® Colesterolo Plus on Serum Lipids, Endothelial Reactivity, Indexes of Non-Alcoholic Fatty Liver Disease and Systemic Inflammation in Healthy Subjects with Polygenic Hypercholesterolemia: The ANEMONE Study. Nutrients 2022, 14, 2099. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic Compounds and Biological Rhythms: Who Takes the Lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Hironao, K.; Mitsuhashi, Y.; Huang, S.; Oike, H.; Ashida, H.; Yamashita, Y. Cacao Polyphenols Regulate the Circadian Clock Gene Expression and through Glucagonnlike Peptidee1 Secretion. J. Clin. Biochem. Nutr. 2020, 67, 53–60. [Google Scholar] [CrossRef]
- Rodríguez, R.M.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Feillet-Coudray, C.; Casas, F.; Colom-Pellicer, M.; Aragonès, G.; Avila-Román, J.; Muguerza, B.; Mulero, M.; et al. Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats. Nutrients 2022, 14, 774. [Google Scholar] [CrossRef]
- Escobar-Martínez, I.; Arreaza-Gil, V.; Muguerza, B.; Arola-Arnal, A.; Bravo, F.I.; Torres-Fuentes, C.; Suárez, M. Administration Time Significantly Affects Plasma Bioavailability of Grape Seed Proanthocyanidins Extract in Healthy and Obese Fischer 344 Rats. Mol. Nutr. Food Res. 2022, 66, 552. [Google Scholar] [CrossRef]
- Türk, D.; Scherer, N.; Selzer, D.; Dings, C.; Hanke, N.; Dallmann, R.; Schwab, M.; Timmins, P.; Nock, V.; Lehr, T. Significant Impact of Time-of-Day Variation on Metformin Pharmacokinetics. Diabetologia 2023, 66, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branecky, K.L.; Niswender, K.D.; Pendergast, J.S. Disruption of Daily Rhythms by High-Fat Diet Is Reversible. PLoS ONE 2015, 10, e0137970. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.M.; Montoya, A.; Pujante, I.M.; Pérez-Sánchez, J.; Calduch-Giner, J.A.; Mancera, J.M.; Moliner, J.; Sánchez-Vázquez, F.J. Acute Stress Response in Gilthead Sea Bream (Sparus aurata L.) Is Time-of-Day Dependent: Physiological and Oxidative Stress Indicators. Chronobiol. Int. 2014, 31, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano, B.M.; Moreno, A.; Tasset, I.; Túnez, I. Impact of Light/Dark Cycle Patterns on Oxidative Stress in an Adriamycin-Induced Nephropathy Model in Rats. PLoS ONE 2014, 9, e97713. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Li, H. Circadian Rhythm in Adipose Tissue: Novel AOX Target for Metabolic and Cardiovascular Diseases. AOXs 2020, 9, 968. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Chronic Consumption of Dietary Proanthocyanidins Modulates Peripheral Clocks in Healthy and Obese Rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Dietary Proanthocyanidins Modulate the Rhythm of BMAL1 Expression and Induce RORα Transactivation in HepG2 Cells. J. Funct. Foods 2015, 13, 336–344. [Google Scholar] [CrossRef]
- Colom-Pellicer, M.; Rodríguez, R.M.; Soliz-Rueda, J.R.; de Assis, L.V.M.; Navarro-Masip, È.; Quesada-Vázquez, S.; Escoté, X.; Oster, H.; Mulero, M.; Aragonès, G. Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption. Nutrients 2022, 14, 2246. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Yang, S.C.; Tseng, H.L.; Hwang, L.L.; Chen, C.T.; Shieh, K.R. Abnormal Expressions of Circadian-Clock and Circadian Clock-Controlled Genes in the Livers and Kidneys of Long-Term, High-Fat-Diet-Treated Mice. Int. J. Obes. 2010, 34, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.Q.; Zhang, D.; Jin, T.; Cai, D.J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic AOX Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Martin, V.; María Sainz, R.; Mayo, J.C.; Antolín, I.; Herrera, F.; Rodríguez, C. Daily Rhythm of Gene Expression in Rat Superoxide Dismutases. Endocr. Res. 2003, 29, 83–95. [Google Scholar] [CrossRef]
- Patel, S.A.; Velingkaar, N.S.; Kondratov, R.V. Transcriptional Control of AOX Defense by the Circadian Clock. Antioxid. Redox Signal 2014, 20, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Wu, W.; Mi, Y.; Shi, R.; Sun, K.; Li, R.; Liu, X.; Liu, X. Tea Polyphenols Direct Bmal1-Driven Ameliorating of the Redox Imbalance and Mitochondrial Dysfunction in Hepatocytes. Food Chem. Toxicol. 2018, 122, 181–193. [Google Scholar] [CrossRef] [PubMed]
- McClean, C.; Davison, G.W. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? AOXs 2022, 11, 256. [Google Scholar] [CrossRef]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 Regulates Core and Stabilizing Circadian Clock Loops, Coupling Redox and Timekeeping in Mus Musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Imaizumi, A.; Komatsu, M.; Daimon, T.; Miyano, H.; Tani, M.; Toda, A.; Yamamoto, H.; et al. Plasma Amino Acid Profile Associated with Fatty Liver Disease and Co-Occurrence of Metabolic Risk Factors. Sci. Rep. 2017, 7, 14485. [Google Scholar] [CrossRef] [Green Version]
- Mardinoglu, A.; Bjornson, E.; Zhang, C.; Klevstig, M.; Söderlund, S.; Ståhlman, M.; Adiels, M.; Hakkarainen, A.; Lundbom, N.; Kilicarslan, M.; et al. Personal Model-assisted Identification of NAD + and Glutathione Metabolism as Intervention Target in NAFLD. Mol. Syst. Biol. 2017, 13, 916. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Xu, R.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. Glycine Protects against High Sucrose and High Fat-Induced Non-Alcoholic Steatohepatitis in Rats. Oncotarget 2016, 7, 80223–80237. [Google Scholar] [CrossRef] [Green Version]
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-Based Treatment Ameliorates NAFLD by Modulating Fatty Acid Oxidation, Glutathione Synthesis, and the Gut Microbiome. Sci. Transl. Med. 2020, 12, eaaz2841. [Google Scholar] [CrossRef]
- Du, K.; Hyun, J.; Premont, R.T.; Choi, S.S.; Michelotti, G.A.; Swiderska-Syn, M.; Dalton, G.D.; Thelen, E.; Rizi, B.S.; Jung, Y.; et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology 2018, 154, 1465–1479.e13. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.; Gu, Y.; Hong, Y.; Sheng, L.; Chen, L.; Zhang, F.; Hou, J.; Zhang, W.; Zhang, Z.; Jia, W.; et al. Vancomycin Pretreatment Attenuates Acetaminophen-Induced Liver Injury through 2-Hydroxybutyric Acid. J. Pharm. Anal. 2020, 10, 560–570. [Google Scholar] [CrossRef]
- Violet, P.C.; Ebenuwa, I.C.; Wang, Y.; Niyyati, M.; Padayatty, S.J.; Head, B.; Wilkins, K.; Chung, S.; Thakur, V.; Ulatowski, L.; et al. Vitamin E Sequestration by Liver Fat in Humans. JCI Insight 2020, 5, e133309. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, K.R.C.; dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night Workers Have Lower Levels of AOX Defenses and Higher Levels of Oxidative Stress Damage When Compared to Day Workers. Sci. Rep. 2019, 9, 4455. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.D.; Woo, K.C.; Cho, S.; Ha, D.C.; Sung, K.J.; Kim, K.T. Rhythmic Control of AANAT Translation by HnRNP Q in Circadian Melatonin Production. Genes. Dev. 2007, 21, 797–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, C.M.; Koronowski, K.B.; Smith, J.G.; Shi, J.; Kunderfranco, P.; Carriero, R.; Chen, S.; Samad, M.; Welz, P.-S.; Zinna, V.M.; et al. Integration of Feeding Behavior by the Liver Circadian Clock Reveals Network Dependency of Metabolic Rhythms. Sci. Adv. 2021, 7, eabi7828. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Santos, R.B.; Fekry, B.; Tamim, Y.M.; Shivshankar, S.; Mohamed, A.M.T.; Baumgartner, C.; Kwok, C.; Gebhardt, C.; Rivera, A.; et al. Cellular and Physiological Circadian Mechanisms Drive Diurnal Cell Proliferation and Expansion of White Adipose Tissue. Nat. Commun. 2021, 12, 3482. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.O.; Yoshitane, H.; Kim, D.W.; Kawakami, S.; Koebis, M.; Nakao, K.; Aiba, A.; Kim, J.K.; Fukada, Y. Rhythmic Transcription of Bmal1 Stabilizes the Circadian Timekeeping System in Mammals. Nat. Commun. 2022, 13, 4652. [Google Scholar] [CrossRef]









| Gene | Accession Number | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| Sod1 | NM_017050.1 | GGTGGTCCACGAGAAACAAG | CAATCACACCACAAGCCAAG |
| Sod2 | NM_017051.2 | AAGGAGCAAGGTCGCTTACA | ACACATCAATCCCCAGCAGT |
| Catalase | NM_012520.2 | GAATGGCTATGGCTCACACA | CAAGTTTTTGATGCCCTGGT |
| GPx1 | NM_030826.4 | TGCAATCAGTTCGGACATC | CACCTCGCACTTCTCAAACA |
| GSR | NM_053906.2 | ATCAAGGAGAAGCGGGATG | GCGTAGCCGTGGATGACT |
| PPIA | NM_017101.1 | TCAAACACAAATGGTTCCCAGT | ATTCCTGGACCCAAAACGCT |
| Glucose (mg/dL) | Triglycerides (mg/dL) | Cholesterol (mg/dL) | NEFA (mg/dL) | |||
|---|---|---|---|---|---|---|
| ZT0 | ZT1 | STD-VH CAF-VH CAF-GSPE | 88.27 (87.04–91.34) 96.47 (91.64–101.04) 95.6 (94.07–106.31) | 60.97 (56.62–68.42) 108.22 (100.66–116.06) + 105.26 (90.13–123.62) | 97.26 (81.37–106.85) 93.83 (88.1–101.71) 95.82 (92.26–106.46) | 19.72 (17.23–24.42) 22.87 (21.66–25.92) 21.81 (20.27–22.31) |
| ZT7 | STD-VH CAF-VH CAF-GSPE | 86.99 (86.13–90.32) 112.14 (105.06–117.92) + 116.47 (111.27–119.8) | 102.7 (102.16–104.32) 331.89 (312.97–338.11) $ 276.22 (251.62–304.86) | 103.11 (96.69–107.59) 128.02 (120.62–141.63) 141.25 (119.07–164.2) | 31.44 (29.57–34.28) 29.81 (28.8–32.16) 34.13 (32.31–35) | |
| ZT13 | STD-VH CAF-VH CAF-GSPE | 86.67 (84.78–91.5) 113.58 (106.59–118.28) $ 99.12 (94.21–101.03) | 54.96 (40–68.09) 184.23 (154.72–234.45) + 103.95 (98.17–110.53) | 91.5 (83.67–95.44) 97.58 (81.47–128.97) 76.53 (69.16–86.97) | 25.64 (15.8–35.22) 31.13 (28.05–35.71) 36.27 (35–36.78) | |
| ZT19 | STD-VH CAF-VH CAF-GSPE | 77.79 (74.75–82.66) 100.9 (94.29–102.81) 102.09 (83.87–115.6) | 51.95 (43.94–58.22) 143.42 (125.17–151.93) + 131.83 (129.07–133.02) | 85.27 (69.73–101.29) 94.01 (91.06–100) 81.19 (71.79–91.94) | 22.47 (19.07–25.64) 27.19 (26.65–29.21) 29.85 (28.54–33.23) | |
| ZT12 | ZT1 | STD-VH CAF-VH CAF-GSPE | 90.75 (90.24–91.04) 101.81 (97.34–108.29) $ 117.61 (116.87–117.75) # | 74.69 (56.62–102.3) 115.13 (110.64–127.41) 181.41 (141.95–221.28) | 128.63 (104.17–144.09) 92.74 (84.58–96.55) 95.6 (87.07–101.21) | 23.7 (20.27–34.9) 26.41 (22.82–30.18) 25.82 (20.65–35.82) |
| ZT7 | STD-VH CAF-VH CAF-GSPE | 90.17 (78.03–99.42) 113.29 (110.84–118.79) + 123.12 (116.91–132.08) | 160.54 (137.3–186.76) 302.7 (278.38–344.59) + 338.38 (302.16–402.97) | 123.74 (114.79–143.19) 111.28 (104.09–129.57) 145.14 (130.93–165.56) | 29.52 (26.3–33.37) 31.25 (30.19–33.8) 34.71 (28.03–41.15) | |
| ZT13 | STD-VH CAF-VH CAF-GSPE | 83.88 (78.75–90.62) 124.38 (108.65–139.85) + 108.81 (100.02–121.72) | 51.01 (47.89–52.96) 219.64 (177.75–230.92) + 145.14 (127.84–200.93) | 102.52 (91.99–111.03) 118.99 (110.34–127.92) 106.86 (102.63–122.46) | 27.56 (24.26–30.59) 33.89 (29.45–37.72) 32.25 (30.4–36.08) | |
| ZT19 | STD-VH CAF-VH CAF-GSPE | 79.61 (74.19–86.42) 119.94 (112.05–132.94) + 95.22 (89.4–112.17) | 65.48 (60.47–70.25) 211.27 (149.74–275.05) + 146.16 (126.32–199.72) | 88.8 (86.05–96.3) 133.02 (104.78–162.27) 112 (94.62–145.82) | 30.96 (28.13–33.85) 30.76 (27.34–33.6) 24.11 (22.67–25.69) |
| Parameter | Group | Period (h) | p | MESOR | Amplitude | p (Amplitude) | Acrophase (h) | p (Acrophase) |
|---|---|---|---|---|---|---|---|---|
| SOD | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.001 ** 0.002 ** <0.001 *** | 11.52 12.30 12.29 | 2.52 1.85 3.62 | <0.001 *** <0.001 *** <0.001 *** | 15.99 15.59 15.08 | <0.001 *** <0.001 *** <0.001 *** |
| Catalase | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.531 0.697 0.088 # | 709.56 714.29 676.71 | 24.36 15.90 55.97 | 0.249 0.389 0.014 * | 16.83 6.27 11.63 | <0.001 *** <0.001 *** <0.001 *** |
| GPx1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.027 * 0.033 * 0.024 * | 2271.60 1897.17 1847.25 | 278.79 290.64 425.05 | 0.002 ** 0.003 ** 0.001 ** | 16.02 19.60 15.09 | <0.001 *** <0.001 *** <0.001 *** |
| GSH | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.001 ** 0.434 0.063 # | 2583.39 2021.05 1744.49 | 591.45 255.83 396.18 | <0.001 *** 0.181 0.008 ** | 4.04 6.95 5.44 | <0.001 *** 0.023 * <0.001 *** |
| Thiols | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.039 * 0.082 # 0.052 # | 94.85 92.03 94.49 | 17.32 12.53 15.92 | 0.004 ** 0.013 * 0.006 ** | 2.78 6.67 6.84 | 0.034 * <0.001 *** <0.001 *** |
| Parameter | Group | Period (h) | p | MESOR | Amplitude | p (Amplitude) | Acrophase (h) | p (Acrophase) |
|---|---|---|---|---|---|---|---|---|
| SOD | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.010 * 0.055 # 0.014 * | 12.05 12.04 11.45 | 1.66 1.34 1.05 | <0.001 *** <0.001 *** <0.001 *** | 15.31 17.36 16.96 | <0.001 *** <0.001 *** <0.001 *** |
| Catalase | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.345 0.045 * 0.061 # | 697.89 687.95 693.49 | 26.90 56.24 46.33 | 0.128 0.004 ** 0.008 ** | 4.21 4.80 5.10 | 0.091 # 0.001 ** <0.001 *** |
| GPx1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.393 0.729 0.993 | 2328.48 1770.59 1751.78 | 143.67 74.43 15.10 | 0.156 0.421 0.906 | 11.79 20.32 12.64 | <0.001 *** <0.001 *** 0.696 |
| GSH | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.020 * 0.189 0.172 | 2487.20 2074.93 1884.68 | 600.67 319.96 298.25 | 0.001 ** 0.051 # 0.045 * | 4.74 4.80 3.83 | <0.001 *** 0.014 * 0.044 * |
| Thiols | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.009 ** <0.001 *** 0.001 ** | 104.30 105.56 108.25 | 21.19 31.44 25.55 | <0.001 *** <0.001 *** <0.001 *** | 5.25 4.23 3.63 | <0.001 *** <0.001 *** <0.001 *** |
| SOD (U SOD/mg Prot) | Catalase (U/mg Prot) | GPx1 (mU GPx1/mg Prot) | GSH (nmol GSH/mg Prot) | Oxidized Proteins (nmol -SH/mg Prot) | |||
|---|---|---|---|---|---|---|---|
| ZT0 | LIGHT PHASE | STD-VH CAF-VH CAF-GSPE | 9.67 (9.3–10.26) 10.88 (10.42–11.66) ++ 9.87 (9.58–10.3) ** | 668.13 (664.70–758.88) 720.95 (691.91–776.14) 642.71 (634.64–678.85) * | 2078.03 (2007.69–2115.74) 1741.21 (1424.32–1995.01) 1410.08 (1302.11–1831.33) | 3057.76 (2989.45–3207.91) 1998.10 (1828.45–2923.07) + 2054.13 (1726.91–2317.81) | 115.98 (90.38–118.84) 93.71 (87.57–120.04) 105.40 (87.03–118.47) |
| DARK PHASE | STD-VH CAF-VH CAF-GSPE | 13.11 (12.73–14.35) && 13.75 (12.62–15.03) && 14.39 (13.67–15.81) && | 722.30 (709.01–748.09) 700.33 (687.36–724.93) 676.06 (648.30–706.40) | 2494.95 (2365.07–2599.67) & 2013.04 (1891.08–2191.33) ++ 2216.50 (1786.01–2372.41) & | 2167.02 (2065.72–2255.08) && 1862.04 (1633.34–2119.29) 1495.00 (1360.02–1699.41) & | 83.76 (78.48–87.65) & 83.69 (79.84–87.68) & 82.92 (78.59–88.16) & | |
| ZT12 | LIGHT PHASE | STD-VH CAF-VH CAF-GSPE | 10.64 (10.02–11.62) 11.13 (10.58–11.67) 10.85 (10.33–11.04) | 725.53 (698.82–744.47) 717.64 (655.40–740.22) 740.96 (686.92–766.97) | 2296.09 (2125.45–2481.63) 1742.62 (1617.97–1920.80) ++ 1806.08 (1607.78–1876.91) | 3005.38 (2741.25–3079.27) 2414.31 (2110.62–2563.82) + 2137.81 (1923.12–2213.25) | 112.93 (109.39–124.75) 130.16 (118.01–135.57) 129.61 (114.16–137.15) |
| DARK PHASE | STD-VH CAF-VH CAF-GSPE | 13.39 (12.17–13.82) && 12.66 (12.19–13.46) & 12.57 (11.24–12.9) & | 662.92 (651.74–690.47) & 647.61 (625.13–668.53) & 656.97 (644.57–679.19) | 2449.82 (2319.24–2529.99) 1767.47 (1636.02–1882.40) ++ 1790.96 (1496.10–2108.56) | 1989.62 (1884.67–2420.08) & 1941.43 (1560.06–2069.69) & 1651.59 (1451.46- 1835.57) | 89.78 (78.92–100.80) && 82.24 (79.21–89.49) &&& 89.53 (77.87–93.04) && |
| Parameter | Group | Period (h) | p | MESOR | Amplitude | p (Amplitude) | Acrophase (h) | p (Acrophase) |
|---|---|---|---|---|---|---|---|---|
| Sod1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.643 0.266 0.021 * | 0.98 1.02 1.04 | 0.08 0.11 0.13 | 0.339 0.086 # 0.001 ** | 22.97 15.35 20.33 | <0.001 *** <0.001 *** <0.001 *** |
| Sod2 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.008 ** 0.146 0.001 ** | 1.05 1.27 1.34 | 0.31 1.27 1.34 | <0.001 *** 0.034 * <0.001 *** | 19.59 18.76 19.84 | <0.001 *** <0.001 *** <0.001 *** |
| Catalase | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.891 0.170 0.911 | 1.04 1.04 0.98 | 0.04 0.12 0.04 | 0.629 0.044 * 0.666 | 14.09 13.43 22.68 | 0.075 # <0.001 *** 0.009 ** |
| GPx1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.367 0.966 0.557 | 0.92 0.86 0.87 | 0.12 0.02 0.07 | 0.141 0.793 0.268 | 1.77 19.93 22.55 | 0.494 0.172 <0.001 *** |
| GSR | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.176 0.943 0.746 | 1.07 1.14 1.11 | 0.14 0.03 0.07 | 0.046 * 0.731 0.439 | 18.37 11.12 19.40 | <0.001 *** <0.001 *** <0.001 *** |
| Parameter | Group | Period (h) | p | MESOR | Amplitude | p (Amplitude) | Acrophase (h) | p (Acrophase) |
|---|---|---|---|---|---|---|---|---|
| Sod1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.095 # 0.047 * 0.084 # | 0.86 0.83 0.74 | 0.24 0.24 0.16 | 0.017 * 0.005 ** 0.014 * | 19.45 19.70 19.49 | <0.001 *** <0.001 *** <0.001 *** |
| Sod2 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.003 ** 0.008 ** 0.002 ** | 1.12 1.35 1.12 | 0.38 0.42 0.34 | <0.001 *** <0.001 *** <0.001 *** | 18.40 20.81 20.28 | <0.001 *** <0.001 *** <0.001 *** |
| Catalase | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.432 0.005 ** 0.309 | 1.20 1.18 0.95 | 0.09 0.18 0.09 | 0.180 <0.001 *** 0.109 | 11.89 21.24 21.93 | <0.001 *** <0.001 *** <0.001 *** |
| GPx1 | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.005 ** 0.100 0.097 # | 1.05 1.12 0.86 | 0.32 0.17 0.11 | <0.001 *** 0.019 * 0.018 * | 6.25 2.77 0.34 | <0.001 *** 0.088# 0.831 |
| GSR | STD-VH CAF-VH CAF-GSPE | 24 24 24 | 0.239 0.216 0.477 | 1.14 1.15 0.97 | 0.14 0.15 0.08 | 0.074 # 0.063 # 0.210 | 17.05 21.17 20.10 | <0.001 *** <0.001 *** <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Espinar, A.J.; Ibarz-Blanch, N.; Soliz-Rueda, J.R.; Bonafos, B.; Feillet-Coudray, C.; Casas, F.; Bravo, F.I.; Calvo, E.; Ávila-Román, J.; Mulero, M. Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats. Antioxidants 2023, 12, 1606. https://doi.org/10.3390/antiox12081606
Cortés-Espinar AJ, Ibarz-Blanch N, Soliz-Rueda JR, Bonafos B, Feillet-Coudray C, Casas F, Bravo FI, Calvo E, Ávila-Román J, Mulero M. Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats. Antioxidants. 2023; 12(8):1606. https://doi.org/10.3390/antiox12081606
Chicago/Turabian StyleCortés-Espinar, Antonio J., Néstor Ibarz-Blanch, Jorge R. Soliz-Rueda, Béatrice Bonafos, Christine Feillet-Coudray, François Casas, Francisca Isabel Bravo, Enrique Calvo, Javier Ávila-Román, and Miquel Mulero. 2023. "Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats" Antioxidants 12, no. 8: 1606. https://doi.org/10.3390/antiox12081606