Different Effect of Vitamin E or Hydroxytyrosol Supplementation to Sow’s Diet on Oxidative Status and Performances of Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethics Statement
2.3. Animals, Experimental Procedures and Diets
2.4. Growth Evaluation
2.5. Sample Collection
2.6. Laboratory Analysis
2.6.1. Antioxidant Enzymes Determination
2.6.2. Tocopherol Quantification in Plasma Samples
2.6.3. TBARs Quantification in Plasma Samples
2.7. Statistical Analysis
3. Results
3.1. Oxidative Status of Sows and Piglets
3.2. Performances and Piglet’s Growth
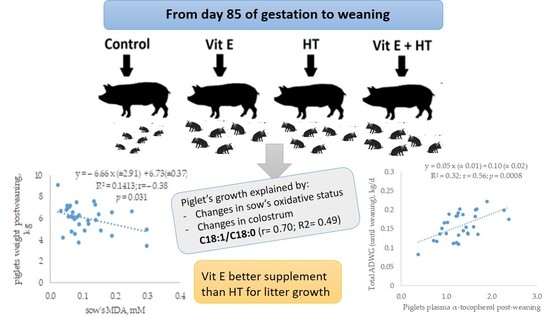
3.3. Relationship between Sows’ Oxidative Status and Piglets’ Growth
3.4. Relationship between Milk Composition and Piglet’s Growth
4. Discussion
4.1. Oxidative Status of Sows and Piglets
4.2. Performances and Piglet’s Growth
4.3. Relationship between Sows’ Oxidative Status or Milk Composition and Piglets’ Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Analysed Composition 1 | CONTROL | VE2 | HXT | HXT + VE |
|---|---|---|---|---|
| Dry matter, % | 90.8 | 89.8 | 91.1 | 91.2 |
| Crude Protein, % | 13.1 | 14.0 | 15.0 | 14.5 |
| Fat, % | 4.3 | 3.6 | 3.8 | 3.7 |
| Ash, % | 6.5 | 6.4 | 6.9 | 6.0 |
| Fiber, % | 4.3 | 4.4 | 4.3 | 4.4 |
| Starch, % | 49.0 | 46.1 | 41.1 | 43.0 |
| Vitamin E, mg/kg | 70.5 | 103.6 | 65.4 | 114.1 |
| Fatty acid composition | ||||
| C14:0 | 0.60 | 0.66 | 0.56 | 0.60 |
| C16:0 | 19.80 | 21.48 | 19.10 | 20.11 |
| C16:1n-9 | 0.12 | 0.15 | 0.12 | 0.12 |
| C16:1n-7 | 0.79 | 0.87 | 0.73 | 0.78 |
| C18:0 | 4.77 | 4.80 | 4.45 | 4.54 |
| C18:1n-9 | 28.97 | 25.26 | 30.33 | 26.94 |
| C18:1n-7 | 1.92 | 1.49 | 1.57 | 1.60 |
| C18:2n-6 | 38.97 | 40.81 | 38.97 | 40.89 |
| C18:3n-3 | 3.04 | 3.44 | 3.15 | 3.36 |
| C20:0 | 0.30 | 0.26 | 0.30 | 0.31 |
| C20:1n-9 | 0.52 | 0.58 | 0.55 | 0.58 |
| ∑SAT | 25.47 | 27.20 | 24.41 | 25.55 |
| ∑MUFA | 32.33 | 28.35 | 33.29 | 30.02 |
| ∑PUFA | 42.01 | 44.25 | 42.11 | 44.25 |
References
- Campos, P.H.R.F.; Silva, B.A.N.; Donzele, J.L.; Oliveira, R.F.M.; Knol, E.F. Effects of sow nutrition during gestation on within-litter birth weight variation: A review. Animal 2012, 6, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Gondret, F.; Lefaucheur, L.; Juin, H.; Louveau, I.; Lebret, B. Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J. Anim. Sci. 2006, 84, 93–103. [Google Scholar] [CrossRef]
- Arnay, M.A.; de Andrés Alvaro, M.Á.; Noguera, C.P.; Estévez, V.R. Análisis de parámetros reproductivos del cerdo ibérico. Primera propuesta para establecer los niveles de referencia. Anaporc Rev. Asoc. Porc. Científica 2011, 8, 40–445. [Google Scholar]
- Wu, G.; Bazer, F.; Wallace, J.; Spencer, T. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Fix, J.S.; Cassady, J.P.; Holl, J.W.; Herring, W.O.; Culbertson, M.S.; See, M.T. Effect of piglet birth weight on survival and quality of commercial market swine. Livest. Sci. 2010, 132, 98–106. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [Green Version]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Pinelli-Saavedra, A. Vitamin E in immunity and reproductive performance in pigs. Reprod. Nutr. Dev. 2003, 43, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Amazan, D.; Rey, A.I.; Fernandez, E.; López-Bote, C.J. Natural vitamin E (d-α-tocopherol) supplementation in drinking water prevents oxidative stress in weaned piglets. Livest. Sci. 2012, 145, 55–462. [Google Scholar]
- Amazan, D.; Cordero, G.; López-Bote, C.J.; Lauridsen, C.; Rey, A.I. Effects of oral micellized natural vitamin E (d-α-tocopherol) vs. synthetic vitamin E (dl-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets. Animal 2014, 8, 410–4419. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, A.; Ghorbel, H.; Bouallegui, Z.; Marrekchi, R.; Isoda, H.; Saydi, S. Oleuropein and hydroxytyrosol protect from bisphenol A effects in livers and kidneys of lactating mother rats and their pups’. Exp. Toxicol. Pathol. 2015, 67, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xiaodong, X.; Ge, S.; Baoming, S.; Anshan, S. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mavromatis, J.; Koptopoulos, G.; Kyriakis, S.C.; Papasteriadis, A.; Saoulidis, K. Effects of α-tocopherol and selenium on pregnant sows and their piglets’ immunity and performance. J. Vet. Med. 1999, 46, 543–553. [Google Scholar]
- Mahan, D.C.; Kim, Y.Y.; Stuart, R.L. 2000 Effect of vitamin E sources (RRR- or -rac-alpha-tocopheryl acetate) and levels on sow reproductive performance, serum, tissue and milk alphatocopherol contents over a five parity period, and the effects on the progeny. J. Anim. Sci. 2000, 78, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Gómez, M.; García-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; González-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR Pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar]
- Leskovec, J.; Rezar, V.; Nemec, A.; Salobir, J.; Levart, A. Antioxidative effects of olive polyphenols compared to vitamin E in piglets fed a diet rich in n-3 PUFA. Animal 2019, 9, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Rey, A.I.; de-Cara, A.; Calvo, L.; Puig, P.; Hechavarría, T. Changes in Plasma Fatty Acids, Free Amino Acids, Antioxidant Defense, and Physiological Stress by Oleuropein Supplementation in Pigs Prior to Slaughter. Antioxidants 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BOE. RD 53/2013, de 21 de octubre por la que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia, Spain. Boletín Off. Del Estado 2013, 252, 34367–34391. [Google Scholar]
- EC. Council Regulation (EC) No 2010/63/CE of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, 276, 33–79. [Google Scholar]
- NRC (National Research Council). Nutrient Requirement of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the alpha- and gamma-tocopherols accumulation in muscle and backfat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Laviano, H.; Gómez, G.; Muñoz, M.; García-Casco, J.; Nuñez, Y.; Escudero, R.; Heras, A.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; et al. Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk. Antioxidants 2023, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, E.; Viteri, F.E. Iron and oxidative stress in pregnancy. J. Nutr. 2003, 133, 1700S–1708S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demchuk, M.; Buchko, O.; Lis, M.W.; Niedziolka, J.W. Performance of the antioxidant protection in blood of highly prolific sows before and after farrowing. Performance of the antioxidant protection in blood of highly prolific sows before and after farrowing. Large Anim. Rev. 2014, 20, 135–139. [Google Scholar]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Michael Nelson, D. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef] [Green Version]
- Vanhees, K.; van Schooten, F.J.; van Doorn, S.B.; van Helden, S.; Munnia, A.; Peluso, M.; Briedé, J.J.; Haenen, G.R.; Godschalk, R.W. Intrauterine exposure to flavonoids modifies antioxidant status at adulthood and decreases oxidative stress-induced DNA damage. Free Radic. Biol. Med. 2013, 57, 154–161. [Google Scholar] [CrossRef]
- Lipiński, K.; Antoszkiewicz, Z.; Mazur-Kuśnirek, M.; Korniewicz, D.; Kotlarczyk, S. The effect of polyphenols on the performance and antioxidant status of sows and piglets. Ital. J. Anim. Sci. 2019, 18, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, Z.; Cao, X.; Zou, T.; You, J.; Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim. Nutr. 2023, 12, 96–107. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytirosol from olive mil waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- Pinelli-Saavedra, A.; Scaife, J.R. Pre- and postnatal transfer of vitamins E and C to piglets in sows supplemented with vitamin E and vitamin C. Liv. Prod. Sci. 2005, 97, 231–240. [Google Scholar] [CrossRef]
- Migdal, W.; Kaczmarczyk, J. Effect to injection of selenium and vitamin E on reproductive performance of sows and Se concentration in sow milk. World Rev. Anim. Prod. 1993, 28, 68–71. [Google Scholar]
- Parraguez, V.H.; Sales, F.; Peralta, O.A.; de los Reyes, M.; Campos, A.; González, J.M.; Peralta, W.; Cabezón, C.; González-Bulnes, A. Maternal supplementation with herbal antioxidants during pregnancy in swine. Antioxidants 2021, 10, 658. [Google Scholar] [PubMed]
- Lauridsen, C.; Danielsen, V. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest. Prod. Sci. 2004, 91, 95–105. [Google Scholar]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalex, J.; Encinas, T.; Astiz, S.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on postnatal growth, metabolism and body composition of the offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunting, A.M.S.; Sakkas, P.; Wellock, I.; Almond, K.; Kyriazakis, I. Once small always small? To what extent morphometric characteristics and post-weaning starter regime affect pig lifetime growth performance. Porc. Health Manag. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kim, S.W. Oxidative stress status and reproductive performance of sows during gestation and lactation under different thermal environments. Asian-Australas J. Anim. Sci. 2020, 33, 722–731. [Google Scholar] [CrossRef]
- de-Cara, A.; Saldaña, B.; Vázquez, P.; Rey, A.I. Dietary Protected Sodium Butyrate and/or Olive leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants 2023, 12, 201. [Google Scholar] [CrossRef]
- Hall, M.E.; Blount, J.D.; Forbes, S.; Royle, N.J. Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct. Ecol. 2010, 24, 365–373. [Google Scholar]
- Decaluwé, R.; Maes, D.; Wuyts, B.; Cools, A.; Piepers, S.; Janssens, G.P.J. Piglets’ colostrum intake associates with daily weight gain and survival until weaning. Livest. Sci. 2014, 162, 185–192. [Google Scholar]
- Le Dividich, J.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Acid. 2005, 143, 469–485. [Google Scholar]
- Picone, G.; Zappaterra, M.; Luise, D.; Timigno, A.; Capozzi, F.; Motta, V.; Davoli, R.; Nani Costa, L.; Bosi, P.; Trevisi, P.J. Metabolomics characterization of colostrum in three sow breeds and its influences on piglets’ survival and litter growth rates. Anim. Sci. Biotech. 2018, 9, 23–35. [Google Scholar]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar]
- Rao, P.S.; Roa, K.S. Fatty acid composition of phospholipids in different regions of developing human fetal brain. Lipids 1973, 8, 374–377. [Google Scholar]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Hojgaard, C.; Bruun, T.S.; Theil, P.K. Impact of milk and nutrient intake of piglets and sow milk composition on piglet growth and body composition at weaning. J. Anim. Sci. 2020, 98, skaa060. [Google Scholar]



| Control 1 | VE 2 | HXT 3 | VE + HXT 4 | VE-30 | VE-100 | HXT-0 | HXT-1.5 | RMSE 5 | p VE 6 | p HXT | p VE + HXT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 110 of gestation | ||||||||||||
| SOD 7, U/mL | 0.39 | 0.39 | 0.58 | 0.44 | 0.48 | 0.42 | 0.39 a | 0.51 b | 0.153 | 0.247 | 0.044 | 0.252 |
| GSHt 8, µM | 1.72 | 1.54 | 2.79 | 3.16 | 2.25 | 2.35 | 1.63 a | 2.97 b | 1.419 | 0.864 | 0.019 | 0.608 |
| GSSG 9, µM | 0.70 | 0.73 | 0.97 | 0.77 | 0.83 | 0.75 | 0.72 | 0.87 | 0.247 | 0.367 | 0.125 | 0.231 |
| Free GSH 10, µM | 1.02 | 0.80 | 1.82 | 2.39 | 1.42 | 1.60 | 0.91 a | 2.11 b | 1.349 | 0.729 | 0.028 | 0.447 |
| α-Tocopherol, µg/mL | 1.40 | 2.25 | 1.54 | 2.28 | 1.47 B | 2.26 A | 1.82 | 1.91 | 0.666 | 0.004 | 0.746 | 0.824 |
| Day 20 of lactation | ||||||||||||
| SOD, U/mL | 0.43 | 0.62 | 0.58 | 0.54 | 0.51 | 0.58 | 0.53 | 0.56 | 0.320 | 0.486 | 0.977 | 0.496 |
| GSHt, µM | 2.55 | 2.93 | 2.05 | 3.18 | 2.30 | 3.05 | 2.74 | 2.61 | 1.596 | 0.256 | 0.651 | 0.399 |
| GSSG, µM | 0.96 | 1.17 | 1.00 | 1.01 | 0.98 | 1.09 | 1.07 | 1.01 | 0.481 | 0.562 | 0.581 | 0.776 |
| Free GSH, µM | 1.70 | 1.76 | 1.05 | 2.17 | 1.37 | 1.96 | 1.78 | 1.61 | 1.231 | 0.265 | 0.620 | 0.186 |
| α-Tocopherol, µg/mL | 1.48 | 3.01 | 1.81 | 2.79 | 1.65 B | 2.90 A | 2.25 | 2.30 | 0.668 | <0.001 | 0.532 | 0.143 |
| MDA 11, mM | 0.13 | 0.09 | 0.08 | 0.09 | 0.11 | 0.09 | 0.11 | 0.09 | 0.063 | 0.384 | 0.220 | 0.245 |
| Control 1 | VE 2 | HXT 3 | VE + HXT 4 | VE-30 | VE-100 | HXT-0 | HXT-1.5 | RMSE 5 | p VE 6 | p HXT | p VE + HXT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sows’ weights | ||||||||||||
| At d 85, kg | 120.86 | 129.09 | 125.20 | 129.75 | 123.03 | 129.42 | 124.97 | 127.47 | 29.307 | 0.309 | 0.690 | 0.769 |
| At farrowing, kg | 113.20 | 120.42 | 113.54 | 123.46 | 113.37 | 121.94 | 116.81 | 118.50 | 26.497 | 0.267 | 0.826 | 0.860 |
| At weaning, kg | 106.40 | 112.50 | 102.73 | 110.85 | 104.57 | 111.67 | 109.45 | 106.79 | 22.850 | 0.281 | 0.685 | 0.878 |
| Piglets’ weights | ||||||||||||
| At birth, kg | 1.24 | 1.39 | 1.38 | 1.45 | 1.31 B | 1.42 A | 1.31 b | 1.42 a | 0.241 | <0.001 | <0.001 | 0.135 |
| At day 7, kg | 2.40 | 2.51 | 2.55 | 2.68 | 2.47 B | 2.60 A | 2.46 b | 2.62 a | 0.434 | 0.010 | 0.001 | 0.842 |
| At day 20, kg | 4.50 | 4.71 | 4.56 | 4.40 | 4.53 | 4.56 | 4.61 | 4.48 | 0.875 | 0.792 | 0.190 | 0.062 |
| At day 28, kg | 5.27 | 5.65 | 5.40 | 5.35 | 5.34 | 5.50 | 5.46 | 5.38 | 1.096 | 0.177 | 0.501 | 0.083 |
| At day 58, kg | 13.82 | 13.20 | 13.49 | 12.87 | 13.66 | 13.03 | 13.51 | 13.18 | 3.690 | 0.267 | 0.553 | 0.999 |
| ADWG (1–7 d) 7 | 0.16 | 0.16 | 0.17 | 0.18 | 0.16 | 0.17 | 0.16 b | 0.17 a | 0.042 | 0.231 | 0.042 | 0.195 |
| ADWG (8–20 d) | 0.14 b | 0.16 a | 0.14 b | 0.14 b | 0.14 | 0.15 | 0.15 a | 0.14 b | 0.039 | 0.137 | 0.001 | 0.017 |
| ADWG (21–28 d) | 0.11 | 0.13 | 0.10 | 0.12 | 0.10 B | 0.13 A | 0.11 | 0.11 | 0.057 | <0.001 | 0.819 | 0.936 |
| ADWG (0–28 d) | 0.14 | 0.15 | 0.14 | 0.14 | 0.14 B | 0.15 A | 0.14 | 0.14 | 0.032 | 0.013 | 0.505 | 0.233 |
| ADWG (29–58 d) | 0.25 | 0.24 | 0.24 | 0.23 | 0.25 | 0.24 | 0.25 | 0.24 | 0.096 | 0.387 | 0.528 | 0.973 |
| Control 1 | VE 2 | HXT 3 | VE + HXT 4 | VE-30 | VE-100 | HXT-0 | HXT-1.5 | RMSE 5 | p VE 6 | p HXT | p VE + HXT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At birth | ||||||||||||
| Biparietal diameter | 4.75 | 4.90 | 4.83 | 5.13 | 4.79 B | 5.01 A | 4.82 b | 4.98 a | 0.369 | <0.001 | <0.001 | 0.060 |
| Body/Head ratio | 0.26 b | 0.28 a | 0.29 a | 0.28 a | 0.27 | 0.28 | 0.27 | 0.28 | 0.042 | 0.042 | 0.007 | 0.003 |
| Occipito-nasal length | 13.10 | 13.83 | 12.99 | 13.83 | 13.05 B | 13.83 A | 13.46 | 13.41 | 0.927 | <0.001 | 0.609 | 0.590 |
| Trunk length | 21.11 c | 22.06 b | 22.66 a | 22.63 a | 21.89 | 22.35 | 21.59 | 22.64 | 1.831 | 0.023 | <0.001 | 0.014 |
| Thoracic perimeter | 23.22 | 23.86 | 24.05 | 24.51 | 23.63 B | 24.19 A | 23.54 b | 24.28 a | 1.748 | 0.004 | <0.001 | 0.631 |
| Abdominal perimeter | 19.52 | 20.61 | 20.39 | 20.87 | 19.96 B | 20.74 A | 20.06 b | 20.63 a | 1.885 | <0.001 | 0.006 | 0.147 |
| Post-weaning | ||||||||||||
| Body weight | 5.67 | 6.49 | 6.13 | 6.11 | 5.90 | 6.30 | 5.90 | 6.12 | 1.289 | 0.134 | 0.776 | 0.169 |
| Body/Head ratio | 7.73 | 8.08 | 7.74 | 8.19 | 7.74 | 8.13 | 7.74 | 7.97 | 0.180 | 0.083 | 0.958 | 0.188 |
| Carcass weight | 3.18 | 3.78 | 3.51 | 3.54 | 3.34 | 3.66 | 3.47 | 3.52 | 0.769 | 0.073 | 0.740 | 0.164 |
| Bowels weight | 1.65 | 1.88 | 1.67 | 1.67 | 1.66 | 1.78 | 1.75 | 1.67 | 0.581 | 0.348 | 0.719 | 0.300 |
| Head weight | 0.73 | 0.80 | 0.79 | 0.75 | 0.76 | 0.78 | 0.76 | 0.77 | 0.127 | 0.432 | 0.702 | 0.096 |
| Loin weight | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.005 | 0.715 | 0.976 | 0.448 |
| Liver weight | 0.18 | 0.21 | 0.20 | 0.21 | 0.19 | 0.21 | 0.21 | 0.20 | 0.052 | 0.215 | 0.482 | 0.205 |
| Kidneys weight | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.009 | 0.374 | 0.324 | 0.278 |
| Gut weight | 0.90 | 1.03 | 0.91 | 0.88 | 0.90 | 0.96 | 0.95 | 0.89 | 0.357 | 0.477 | 0.653 | 0.236 |
| Biparietal diameter | 5.97 | 6.08 | 6.25 | 6.09 | 6.11 | 6.08 | 6.12 | 6.17 | 0.294 | 1.000 | 0.084 | 0.176 |
| Occipito-nasal length | 15.94 | 15.95 | 16.30 | 16.24 | 16.12 | 16.09 | 16.22 | 16.27 | 0.986 | 0.827 | 0.171 | 0.827 |
| Trunk length | 42.25 | 43.75 | 41.65 | 42.24 | 41.95 | 42.99 | 42.02 | 41.94 | 4.497 | 0.291 | 0.478 | 0.551 |
| Thoracic perimeter | 37.94 | 39.55 | 39.32 | 39.07 | 38.63 | 39.31 | 39.27 | 39.20 | 3.487 | 0.199 | 0.727 | 0.229 |
| Abdominal perimeter | 29.91 | 31.85 | 31.34 | 31.80 | 30.62 | 31.83 | 31.90 | 31.57 | 3.278 | 0.168 | 0.356 | 0.221 |
| Piglets Measurements Post-Weaning | Sow’s GSSG 2 | Sow’s Plasma VE | Sow’s Plasma MDA 3 |
|---|---|---|---|
| ADWG 1 | −0.32 | 0.36 b | −0.40 b |
| Body weight | −0.41 b | 0.17 | −0.37 |
| Carcass weight | −0.35 | 0.31 | −0.32 |
| Bowels weight | −0.30 | −0.06 | −0.29 |
| Loin weight | −0.34 | −0.23 | −0.40 b |
| Head weight | −0.39 b | 0.00 | −0.47 a |
| Liver weight | −0.39 b | 0.08 | −0.38 b |
| Kidneys weight | −0.35 | 0.02 | −0.40 b |
| Gut weight | −0.32 | −0.13 | −0.25 |
| Biparietal diameter | −0.48 a | 0.13 | −0.45 b |
| Occipito-nasal length | −0.46 b | −0.13 | −0.38 b |
| Trunk length | −0.33 | 0.19 | −0.26 |
| Thoracic perimeter | −0.41 b | 0.11 | −0.35 |
| Abdominal perimeter | −0.41 b | 0.08 | −0.35 |
| Intercept | s.d. 12 | Slope | s.d. | Variable x | r | R2 | p Linear 13 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Colostrum Fatty Acid | ||||||||||
| ADWG 11, kg/d (0–28 d) | 0.05 | ± | 0.03 | 0.02 | ± | 0.01 | C16:1n-7 | 0.57 | 0.33 | 0.002 |
| 0.28 | ± | 0.04 | −0.02 | ± | 0.01 | C18:0 | −0.62 | 0.38 | 0.001 | |
| 0.05 | ± | 0.03 | 0.03 | ± | 0.01 | C18:1n-7 | 0.48 | 0.23 | 0.012 | |
| 0.50 | ± | 0.08 | −0.01 | ± | 0.00 | ∑SAT 1 | −0.69 | 0.47 | 0.000 | |
| 0.04 | ± | 0.02 | 0.69 | ± | 0.17 | C16:1n-7/C16:0 | 0.63 | 0.40 | 0.001 | |
| −0.88 | ± | 0.21 | 1.17 | ± | 0.25 | C18:1/C18:0 | 0.70 | 0.49 | <0.001 | |
| −0.27 | ± | 0.11 | 0.68 | ± | 0.18 | Δ-9-desaturase 2 | 0.61 | 0.37 | 0.001 | |
| 0.07 | ± | 0.03 | 2.60 | ± | 1.11 | Δ-6-desaturase 3 | 0.43 | 0.19 | 0.028 | |
| 0.34 | ± | 0.07 | −0.93 | ± | 0.30 | Elongase 18–16 4 | −0.52 | 0.27 | 0.005 | |
| Loin weight, g | −24.42 | ± | 14.91 | 41.05 | ± | 18.32 | C20:1/C20:0 | 0.40 | 0.16 | 0.034 |
| Liver weight, g | 24.24 | ± | 83.93 | 9.44 | ± | 4.52 | Elongase 16–14 5 | 0.38 | 0.14 | 0.047 |
| 251.93 | ± | 20.68 | −4156.73 | ± | 1423.14 | Elongase 20–18 6 | −0.50 | 0.25 | 0.007 | |
| −249.25 | ± | 158.21 | 551.14 | ± | 194.49 | C20:1/C20:0 | 0.49 | 0.24 | 0.009 | |
| Gut weight, g | 1160.90 | ± | 137.91 | −19,594.60 | ± | 9492.35 | Elongase 20–18 | −0.38 | 0.14 | 0.049 |
| −1346.78 | ± | 1039.76 | 2776.78 | ± | 1278.13 | C20:1/C20:0 | 0.39 | 0.15 | 0.039 | |
| Occipital nasal length, cm | 12.11 | ± | 1.74 | 0.20 | ± | 0.09 | ∑PUFA | 0.41 | 0.17 | 0.032 |
| 12.32 | ± | 1.73 | 0.21 | ± | 0.10 | ∑n-6 | 0.39 | 0.15 | 0.040 | |
| 12.71 | ± | 1.13 | 2.87 | ± | 0.96 | ∑n-3 7 | 0.51 | 0.26 | 0.006 | |
| Trunk length, cm | 16.35 | ± | 11.78 | 0.51 | ± | 0.24 | ∑MUFA 8 | 0.39 | 0.15 | 0.041 |
| 16.35 | ± | 11.78 | 0.51 | ± | 0.24 | ∑PUFA 9 | 0.39 | 0.15 | 0.041 | |
| 58.29 | ± | 7.53 | −0.93 | ± | 0.42 | ∑n-6 10 | 0.40 | 0.16 | 0.035 | |
| Thoracic perimeter, cm | 15.75 | ± | 9.97 | 27.91 | ± | 12.26 | C20:1/C20:0 | 0.41 | 0.17 | 0.031 |
| Abdominal perimeter, cm | 20.82 | ± | 4.39 | 0.56 | ± | 0.24 | Elongase 16–14 | 0.43 | 0.18 | 0.026 |
| 9.37 | ± | 9.51 | 26.44 | ± | 11.69 | C20:1/C20:0 | 0.41 | 0.16 | 0.032 |
| Intercept | s.d. 1 | Slope | s.d. | Variable x | r | R2 | p Linear 2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 20 Milk | ||||||||||
| Piglet’s weight postweaning, kg | 4.61 | ± | 0.62 | 61.96 | ± | 24.78 | Elongase 20–18 3 | 0.45 | 0.21 | 0.020 |
| Piglet’s weight postweaning, kg | 11.03 | ± | 2.53 | −6.49 | ± | 3.29 | C20:1/C20:0 | 0.37 | 0.14 | 0.060 |
| Trunk length, cm | 38.91 | ± | 1.64 | 140.85 | ± | 63.38 | Elongase 20–18 | 0.41 | 0.17 | 0.036 |
| Abdominal perimeter, cm | 21.32 | ± | 4.80 | 13.88 | ± | 6.63 | C18:3 n-3 | 0.39 | 0.15 | 0.047 |
| Head weight, kg | 0.63 | ± | 0.06 | 5.94 | ± | 2.14 | Elongase 20–18 | 0.49 | 0.24 | 0.011 |
| Gut weight, kg | −0.39 | ± | 0.50 | 1.84 | ± | 0.69 | C18:3 n-3 | 0.49 | 0.24 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, G.; Laviano, H.D.; García-Casco, J.M.; Escudero, R.; Muñoz, M.; Heras-Molina, A.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; Rey, A.I. Different Effect of Vitamin E or Hydroxytyrosol Supplementation to Sow’s Diet on Oxidative Status and Performances of Weaned Piglets. Antioxidants 2023, 12, 1504. https://doi.org/10.3390/antiox12081504
Gómez G, Laviano HD, García-Casco JM, Escudero R, Muñoz M, Heras-Molina A, González-Bulnes A, Óvilo C, López-Bote C, Rey AI. Different Effect of Vitamin E or Hydroxytyrosol Supplementation to Sow’s Diet on Oxidative Status and Performances of Weaned Piglets. Antioxidants. 2023; 12(8):1504. https://doi.org/10.3390/antiox12081504
Chicago/Turabian StyleGómez, Gerardo, Hernan D. Laviano, Juan M. García-Casco, Rosa Escudero, María Muñoz, Ana Heras-Molina, Antonio González-Bulnes, Cristina Óvilo, Clemente López-Bote, and Ana I. Rey. 2023. "Different Effect of Vitamin E or Hydroxytyrosol Supplementation to Sow’s Diet on Oxidative Status and Performances of Weaned Piglets" Antioxidants 12, no. 8: 1504. https://doi.org/10.3390/antiox12081504