Sour Cherry Pomace Valorization as a Bakery Fruit Filling: Chemical Composition, Bioactivity, Quality and Sensory Properties
Abstract
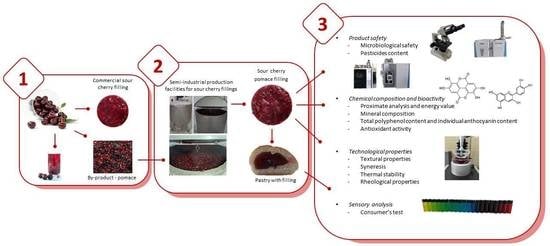
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Filling Production
2.1.2. Baking Process
2.2. Product Safety
2.2.1. Microbiological Safety
2.2.2. Pesticides Content
2.3. Chemical Composition and Bioactivity
2.3.1. Proximate Analysis
2.3.2. Mineral Composition
2.3.3. Total Polyphenol Content and Individual Anthocyanin Content
2.3.4. Antioxidant Activity
2.4. Quality Properties
2.4.1. Textural Properties
2.4.2. Rheological Properties
2.4.3. Syneresis
2.4.4. Thermal Stability
2.5. Questionnaire and Sensory Testing
2.6. Statistical Analysis
3. Results
3.1. Semi-Industrial Processing of Sour Cherry Bakery Fillings
3.2. Product Safety
3.3. Chemical Composition and Bioactivity
3.4. Quality Properties
3.5. Questionnaire and Sensory Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, Crops and Livestock Products Database. Available online: https://www.fao.org/statistics/en/ (accessed on 22 March 2023).
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Bilek, S.E.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials–A Review. Crit. Rev. Food Sci. Nutr. 2018, 59, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents–Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2021, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Cropotova, J.; Tylewicz, U.; Dellarosa, N.; Laghi, L.; Romani, S.; Rosa, M.D. Effect of freezing on microstructure and degree of syneresis in differently formulated fruit fillings. Food Chem. 2016, 195, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, A.; Varela, P.; Sanz, T.; Fiszman, S. Formulating fruit fillings. Freezing and baking stability of a tapioca starch–pectin mixture model. Food Hydrocoll. 2014, 40, 203–213. [Google Scholar] [CrossRef]
- Nalawade, T.; Chavan, R.S.; Joshi, A. Rheological characterization of fruit fillings. In Dairy Engineering-Advanced Technologies and Their Applications; Meghwal, M., Goyal, M.R., Chavan, R.S., Eds.; Taylor & Francis: Waretown, NJ, USA, 2017; pp. 37–52. [Google Scholar] [CrossRef]
- Carcelli, A.; Albertini, A.; Vittadini, E.; Carini, E. A fibre syrup for the sugar reduction in fruit filling for bakery application. Int. J. Gastron. Food Sci. 2022, 28, 100545. [Google Scholar] [CrossRef]
- Young, N.W.; Kappel, G.; Bladt, T. A polyuronan blend giving novel synergistic effects and bake-stable functionality to high soluble solids fruit fillings. Food Hydrocoll. 2003, 17, 407–418. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Fiszman, S. Fruit fillings development: A multiparametric approach. LWT 2015, 61, 564–572. [Google Scholar] [CrossRef]
- Cropotova, J.; Tylewicz, U.; Rocculi, P.; Popel, S.; Rosa, M.D. Thermal properties of fruit fillings as a function of different formulations. Food Struct. 2017, 14, 85–94. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella-Part 1 Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-1:2017; Microbiology of the Food Chain-Horizontal Method for the Detection and ENumeration of Listeria monocytogenes and of Listeria spp.-Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli-Part 2: Colony-Count Technique at 44 Degrees C Using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 21528-2: 2017; Microbiology of the Food Chain—Horizontal Method for the Detection and ENumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with water activity less than or Equal to 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Kecojević, I.; Đekić, S.; Lazović, M.; Mrkajić, D.; Baošić, R.; Lolić, A. Evaluation of LC-MS/MS methodology for determination of 179 multi-class pesticides in cabbage and rice by modified QuEChERS extraction. Food Control. 2021, 123, 107693. [Google Scholar] [CrossRef]
- AOAC. International A: Official Methods of Analysis of the AOAC International; AOAC: Arlington County, VA, USA, 2000. [Google Scholar]
- Službeni list SFRJ 29/83. Pravilnik o metodama uzimanja uzoraka i vršenja hemijskih i fizičkih analiza radi kontrole kvaliteta proizvoda od voća i povrća. Službeni list, Beograd, Socialist Federal Republic of Yugoslavia 1983.
- Perović, J. Razvoj Novog Bezglutenskog Funkcionalnog Flips Proizvoda Oplemenjenog Korenom Cikorije (Cichorium intybus L.); University of Novi Sad: Novi Sad, Serbia, 2022. [Google Scholar]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Takači, A.; Kaplan, M.; Teslić, N.; Zeković, Z.; Lazarević, I.; Pavlić, B. Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization. Foods 2022, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Belović, M.; Torbica, A.; Pajić-Lijaković, I.; Mastilović, J. Development of low calorie jams with increased content of natural dietary fibre made from tomato pomace. Food Chem. 2017, 237, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Nategh, N.A.; Anvar, A.; Dalvand, M.J. Detection of Acetamiprid residue in sour cherry in different degrees of maturity using an electronic nose. J. Agric. Eng. 2020, 43, 235–254. [Google Scholar] [CrossRef]
- Balkan, T.; Kara, K. Determination of Pesticide Residues in Sour Cherry used in the Sour Fruit Juice Production in Tokat provinces. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 106–110. [Google Scholar] [CrossRef]
- Vakula, A.; Horecki, A.T.; Pavlić, B.; Jokanović, M.; Ognjanov, V.; Milović, M.; Teslić, N.; Parpinello, G.; Decleer, M.; Šumić, Z. Application of different techniques on stone fruit (Prunus spp.) drying and assessment of physical, chemical and biological properties: Characterization of dried fruit properties. J. Food Process. Preserv. 2020, 45, 15158. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Djilas, S.; Petrović, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef]
- Krulj, J.; Pezo, L.; Kojić, J.; Solarov, M.B.; Teslić, N. Quality evaluation of cold-pressed oils and semi-defatted cake flours obtained on semi-industrial scale. J. Food Nutr. Res. 2021, 60, 217–228. [Google Scholar]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. African J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: Insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Pedisić, S.; Dragović-Uzelac, V.; Levaj, B.; Škevin, D. Effect of maturity and geographical region on anthocyanin content of sour cherries (Prunus cerasus var. marasca). Food Technol. Biotechnol. 2010, 48, 86–93. [Google Scholar]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crop. Prod. 2013, 51, 279–2888. [Google Scholar] [CrossRef]
- Repajić, M.; Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Kušt, J.; Čošić, Z.; Levaj, B. Influence of cultivar and industrial processing on polyphenols in concentrated sour cherry (Prunus cerasus L.) juic. Food Technol. Biotechnol. 2015, 53, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Shivhare, U. Rheological, textural, micro-structural and sensory properties of mango jam. J. Food Eng. 2010, 100, 357–365. [Google Scholar] [CrossRef]
- Basu, S.; Shivhare, U.S. Rheological, Textural, Microstructural, and Sensory Properties of Sorbitol-Substituted Mango Jam. Food Bioprocess Technol. 2012, 6, 1401–1413. [Google Scholar] [CrossRef]
- Culetu, A.; Manolache, F.; Duta, D. Exploratory Study of Physicochemical, Textural and Sensory Characteristics of Sugar-Free Traditional Plum Jams. J. Texture Stud. 2014, 45, 138–147. [Google Scholar] [CrossRef]
- Kosiorowska, A.; Pietrzyk, S.; Pająk, P.; Socha, R. The effect of the addition of gold flax (Linum usitatissimum L.) and chia seeds (Salvia hispanica L.) on the physicochemical and antioxidant properties of cranberry jams. Eur. Food Res. Technol. 2022, 248, 2865–2876. [Google Scholar] [CrossRef]
- Hosseini, S.; Parastouei, K.; Khodaiyan, F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Toker, O.S.; Polat, B.; Arici, M.; Yilmaz, M.T. Bioactive and rheological properties of rose hip marmalade. J. Food Sci. Technol. 2015, 52, 6465–6474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javanmard, M.; Chin, N.; Yusof, Y.; Endan, J. Application of sago starch as a gelling agent in jam. CyTA-J. Food 2012, 10, 275–286. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Genovese, D.B. Pectin Gels Enriched with Dietary Fibre for the Development of Healthy Confectionery Jams. Food Technol. Biotechnol. 2018, 56, 441–453. [Google Scholar] [CrossRef]
- Liu, J.; Bech, A.C.; Waehrens, S.S.; Bredie, W.L. Perception and liking of yogurts with different degrees of granularity in relation to ethnicity, preferred oral processing and lingual tactile acuity. Food Qual. Preference 2020, 90, 104158. [Google Scholar] [CrossRef]



| Microorganism | Sour Cherry | Sour Cherry Pomace | CSCF | SCPF |
|---|---|---|---|---|
| Salmonella spp./25 g | n.d. | n.d. | n.d. | n.d. |
| Listeria monocytogenes/25 g | n.d. | n.d. | n.d. | n.d. |
| E. coli (cfu/g) | <10 | <10 | <10 | <10 |
| Enterobacteriaceae (cfu/g) | 100 | 110 | <10 | <10 |
| Yeasts and molds (cfu/g) | 200 | 220 | <10 | <10 |
| Pesticide (mg/kg fw) | ||||
| Acetamiprid * | n.a. | n.a. | 0.006 ± 0.00 a | 0.035 ± 0.001 b |
| All other pesticides | n.a. | n.a. | <0.005 | <0.005 |
| Proximate Composition (g/100 g fw) | CSCF | SCPF |
|---|---|---|
| Moisture | 52.62 ± 0.02 b | 48.36 ± 0.03 a |
| Ash | 0.31 ± 0.02 a | 0.30 ± 0.02 a |
| Carbohydrates of which | 45.76 ± 0.70 a | 46.87 ± 0.65 a |
| Sugars | 28.81 ± 0.50 a | 29.72 ± 0.05 b |
| Dietary fibers | 0.79 ± 0.01 a | 3.79 ± 0.02 b |
| Proteins | 0.49 ± 0.03 a | 0.59 ± 0.03 b |
| Fats | 0.03 ± 0.00 a | 0.04 ± 0.00 a |
| Salt | 0.04 ± 0.01 a | 0.05 ± 0.01 a |
| Total soluble solids (°Brix) | 43.38 ± 0.03 a | 44.86 ± 0.07 b |
| Mineral composition (mg/kg fw) | ||
| Zn | 0.99 ± 0.05 a | 1.44 ± 0.02 b |
| Cu | 0.31 ± 0.01 a | 0.54 ± 0.02 b |
| Fe | 2.87 ± 0.04 a | 3.83 ± 0.09 b |
| Mg | 62.64 ± 1.70 a | 85.77 ± 2.01 b |
| K | 1107.40 ± 7.84 a | 863.65 ± 5.06 b |
| Na | 164.63 ± 2.03 a | 206.72 ± 3.94 b |
| Ca | 97.37 ± 1.08 a | 173.03 ± 2.80 b |
| Mn | 0.41 ± 0.02 a | 0.42 ± 0.01 a |
| Polyphenols composition and antioxidant activity | ||
| Total polyphenol content (mg GAE/100 g fw) | 140.73 ± 3.59 a | 152.40 ± 6.39 a |
| DPPH (µmol TE/g fw) | 20.65 ± 1.00 a | 21.33 ± 0.24 a |
| Cyanidin-3-O-sophoroside (mg CGE/100 g fw) | 0.77 ± 0.03 | n.d. |
| Cyanidin-3-O-glucosyl-rutinoside (mg CGE/100 g fw) | 7.89 ± 0.19 b | 5.29 ± 0.06 a |
| Cyanidin-3-O-rutinoside (mg CGE/100 g fw) | 3.41 ± 0.02 b | 2.29 ± 0.08 a |
| ∑ Anthocyanins (mg CGE/100 g fw) | 12.09 ± 0.17 b | 7.58 ± 0.13 a |
| Parameter | CSCF | SCPF |
|---|---|---|
| Hardness/firmness (g force) * | 319.90 ± 22.76 a | 354.88 ± 10.25 b |
| Work of shear (g s) * | 292.27 ± 46.06 a | 328.53 ± 29.88 a |
| Stickiness (g force) * | −209.42 ± 17.96 b | −251.54 ± 7.25 a |
| Work of adhesion (g s) * | −144.21 ± 5.85 a | −142.64 ± 5.75 a |
| Syneresis | n.d. | n.d. |
| Diameter of filling after baking at 100 °C (cm) | 5.12 ± 0.19 a | 4.95 ± 0.06 a |
| Parameter | Temperature (°C) | CSCF | SCPF |
|---|---|---|---|
| G′ (Pa) | 25 | 919.4 ± 19.7 a | 1170 ± 45.6 b |
| 60 | 867.6 ± 13.5 a | 1159 ± 30.3 b | |
| 90 | 797.9 ± 2.5 a | 1029 ± 15.5 b | |
| 60 | 903.9 ± 20.4 a | 1181 ± 30.5 b | |
| 25 | 884.8 ± 25.6 a | 1339 ± 31.7 b | |
| G″ (Pa) | 25 | 155.4 ± 10.2 a | 218.4 ± 23.5 b |
| 60 | 174.4 ± 12.9 a | 227.2 ± 6.5 b | |
| 90 | 149.7 ± 0.6 a | 224.9 ± 10.2 b | |
| 60 | 183.4 ± 7.6 a | 205.9 ± 19.6 b | |
| 25 | 174.5 ± 18.5 a | 236.9 ± 19.5 b |
| CSCF | SCPF | |
|---|---|---|
| Appearance | 4.39 ± 0.74 a | 4.12 ± 0.78 a |
| Odor | 4.02 ± 0.84 a | 4.08 ± 0.74 a |
| Taste | 4.12 ± 0.78 a | 4.25 ± 0.63 a |
| Aroma | 4.06 ± 0.84 a | 4.18 ± 0.63 a |
| Texture | 4.00 ± 0.90 a | 3.96 ± 0.71 a |
| Average score | 4.12 ± 0.70 a | 4.12 ± 0.83 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teslić, N.; Kojić, J.; Đermanović, B.; Šarić, L.; Maravić, N.; Pestorić, M.; Šarić, B. Sour Cherry Pomace Valorization as a Bakery Fruit Filling: Chemical Composition, Bioactivity, Quality and Sensory Properties. Antioxidants 2023, 12, 1234. https://doi.org/10.3390/antiox12061234
Teslić N, Kojić J, Đermanović B, Šarić L, Maravić N, Pestorić M, Šarić B. Sour Cherry Pomace Valorization as a Bakery Fruit Filling: Chemical Composition, Bioactivity, Quality and Sensory Properties. Antioxidants. 2023; 12(6):1234. https://doi.org/10.3390/antiox12061234
Chicago/Turabian StyleTeslić, Nemanja, Jovana Kojić, Branislava Đermanović, Ljubiša Šarić, Nikola Maravić, Mladenka Pestorić, and Bojana Šarić. 2023. "Sour Cherry Pomace Valorization as a Bakery Fruit Filling: Chemical Composition, Bioactivity, Quality and Sensory Properties" Antioxidants 12, no. 6: 1234. https://doi.org/10.3390/antiox12061234