Antioxidative Effects of Black Currant and Cornelian Cherry Juices in Different Tissues of an Experimental Model of Metabolic Syndrome in Rats
Abstract
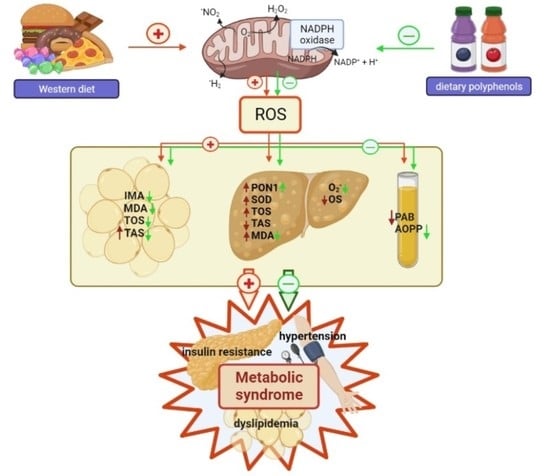
:1. Introduction
2. Materials and Methods
2.1. Juice Sample Preparation
2.2. HPLC–MS/MS Determination of Phenolic Compounds in Juice Samples
2.3. Determination of Total Phenol, Flavan-3-ol and Anthocyanin Contents in Juice Samples
2.4. Animals and Treatment
2.5. Blood and Tissue Sample Preparation
2.6. Oxidative Stress Parameters
2.6.1. PAB Determination
2.6.2. SHG Determination
2.6.3. IMA Determination
2.6.4. MDA Determination
2.6.5. PON1 Determination
2.6.6. SOD Determination
2.6.7. Superoxide Anion Radical Determination
2.6.8. TAS Determination
2.6.9. TOS Determination
2.7. Statistical Analysis
3. Results
3.1. Polyphenols Concentration in Juice Samples
3.2. Consumption of CC and BC Juices
3.3. Body Weight and Visceral Fat Percentage in Rats
3.4. Oxidative Stress Parameters in the Plasma
3.5. Oxidative Stress Parameters in the Liver
3.6. Oxidative Stress Parameters in Adipose Tissue
3.7. Oxy Scores in Different Rats’ Tissues
3.8. Visceral Fat Percentage, Weight, and Oxidative Stress Parameters Correlation
3.9. Multiple Linear Regression Analysis
4. Discussion
BC and CC Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, T.; Xie, Y.; Li, N.; Liu, Y.; Wen, J.; Zhang, M.; Feng, W.; Huang, J.; Guo, Y.; et al. Clitoria Ternatea Blue Petal Extract Protects against Obesity, Oxidative Stress, and Inflammation Induced by a High-Fat, High-Fructose Diet in C57BL/6 Mice. Food Res. Int. 2022, 162, 112008. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2020, 2019, 6175804. [Google Scholar] [CrossRef]
- Vasiljevic, D.; Veselinovic, M.; Jovanovic, M.; Jeremic, N.; Arsic, A.; Vucic, V.; Lucic-tomic, A.; Zivanovic, S. Evaluation of the Effects of Different Supplementation on Oxidative Status in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2016, 35, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Dias Martins, A.M.; Dias, D.M.; Carvalho, C.W.P.; Martino, H.S.D. Germinated Millet Flour (Pennisetum glaucum (L.) R. Br.) Reduces Inflammation, Oxidative Stress, and Liver Steatosis in Rats Fed with High-Fat High-Fructose Diet. J. Cereal Sci. 2021, 99, 103207. [Google Scholar] [CrossRef]
- Kojadinovic, M.; Glibetic, M.; Vucic, V.; Popovic, M.; Vidovic, N.; Debeljak-Martacic, J.; Arsic, A. Short-Term Consumption of Pomegranate Juice Alleviates Some Metabolic Disturbances in Overweight Patients with Dyslipidemia. J. Med. Food 2021, 24, 925–933. [Google Scholar] [CrossRef]
- Williamson, G. The Role of Polyphenols in Modern Nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Slavko, K.; Sandra, B. Antioxidant Capacity of Cornelian Cherry (Cornus mas L.)—Comparison between Permanganate Reducing Antioxidant Capacity and Other Antioxidant Methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The Health Benefits of Blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Mohammadi, K.; Alizadeh Sani, M.; Nattagh-Eshtivani, E.; Yaribash, S.; Rahmani, J.; Shokrollahi Yancheshmeh, B.; Julian McClements, D. A Systematic Review and Meta-Analysis of the Impact of Cornelian Cherry Consumption on Blood Lipid Profiles. Food Sci. Nutr. 2021, 9, 4629–4638. [Google Scholar] [CrossRef]
- Haswell, C.; Ali, A.; Page, R.; Hurst, R.; Rutherfurd-Markwick, K. Potential of Beetroot and Blackcurrant Compounds to Improve Metabolic Syndrome Risk Factors. Metabolites 2021, 11, 338. [Google Scholar] [CrossRef]
- Bursac, S.; Brdovcak, M.C.; Donati, G.; Volarevic, S. Activation of the Tumor Suppressor P53 upon Impairment of Ribosome Biogenesis. Biochim. Biophys. Acta—Mol. Basis Dis. 2014, 1842, 817–830. [Google Scholar] [CrossRef]
- Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants 2022, 11, 1595. [Google Scholar] [CrossRef]
- Attard, E. A Rapid Microtitre Plate Folin-Ciocalteu Method for the Assessment of Polyphenols. Cent. Eur. J. Biol. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of Total Procyanidins in Selected Chocolate and Confectionery Products Using DMAC. J. AOAC Int. 2010, 93, 89–96. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef]
- Ranković, S.; Popović, T.; Martačić, J.D.; Petrović, S.; Tomić, M.; Ignjatović, Đ.; Tovilović-Kovačević, G.; Glibetić, M. Liver Phospholipids Fatty Acids Composition in Response to Different Types of Diets in Rats of Both Sexes. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Kotur-Stevuljevic, J.; Bogavac-Stanojevic, N.; Jelic-Ivanovic, Z.; Stefanovic, A.; Gojkovic, T.; Joksic, J.; Sopic, M.; Gulan, B.; Janac, J.; Milosevic, S. Oxidative Stress and Paraoxonase 1 Status in Acute Ischemic Stroke Patients. Atherosclerosis 2015, 241, 192–198. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A Novel Assay for Cobalt-Albumin Binding and Its Potential as a Marker for Myocardial Ischemia—A Preliminary Report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Early Measurement of Systemic Lipid Peroxidation Products in the Plasma of Major Blunt Trauma Patients. J. Trauma—Inj. Infect. Crit. Care 1991, 31, 32–35. [Google Scholar]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Baralić, K.; Jorgovanović, D.; Živančević, K.; Buha Djordjević, A.; Antonijević Miljaković, E.; Miljković, M.; Kotur-Stevuljević, J.; Antonijević, B.; Đukić-Ćosić, D. Combining in Vivo Pathohistological and Redox Status Analysis with in Silico Toxicogenomic Study to Explore the Phthalates and Bisphenol A Mixture-Induced Testicular Toxicity. Chemosphere 2021, 267, 129296. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Fouret, G.; Gaillet, S.; Lecomte, J.; Bonafos, B.; Djohan, F.; Barea, B.; Badia, E.; Coudray, C.; Feillet-Coudray, C. 20-Week Follow-up of Hepatic Steatosis Installation and Liver Mitochondrial Structure and Activity and Their Interrelation in Rats Fed a High-Fat-High-Fructose Diet. Br. J. Nutr. 2018, 119, 368–380. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; et al. Long-Term Measures of Dyslipidemia, Inflammation, and Oxidative Stress in Rats Fed a High-Fat/High-Fructose Diet. Lipids 2019, 54, 81–97. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Moroni, C.; Savino, S.; Liuzzi, A.; Balzola, F.; Bicchiega, V. Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese Females. J. Clin. Endocrinol. Metab. 2005, 90, 1728–1733. [Google Scholar] [CrossRef]
- Adhe-Rojekar, A.; Mogarekar, M.R.; Rojekar, M.V. Paraoxonase Activity in Metabolic Syndrome in Children and Adolescents. Casp. J. Intern. Med. 2018, 9, 116–120. [Google Scholar] [CrossRef]
- Tisato, V.; Romani, A.; Tavanti, E.; Melloni, E.; Milani, D.; Bonaccorsi, G.; Sanz, J.M.; Gemmati, D.; Passaro, A.; Cervellati, C. Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants 2019, 8, 287. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human Paraoxonase-1 (PON1): Gene Structure and Expression, Promiscuous Activities and Multiple Physiological Roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef]
- Rajkovic, M.G.; Rumora, L.; Barisic, K. The Paraoxonase 1, 2 and 3 in Humans. Biochem. Med. 2011, 21, 122–130. [Google Scholar] [CrossRef]
- Yigittürk, O.; Turgay, F.; Kızıldağ, S.; Özsoylu, D.; Balcı, G.A. Do PON1-Q192R and PON1-L55M Polymorphisms Modify the Effects of Hypoxic Training on Paraoxonase and Arylesterase Activity? J. Sport Health Sci. 2023, 12, 266–274. [Google Scholar] [CrossRef]
- Wu, S.; Kor, C.; Chen, T.; Liu, K.; Shih, K.; Su, W.; Wu, H. Relationships between Serum Uric Acid, Malondialdehyde Levels, and Carotid Intima-Media Thickness in the Patients with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 6859757–6859759. [Google Scholar] [CrossRef]
- Sato, A.; Shiraishi, Y.; Kimura, T.; Osaki, A.; Kagami, K.; Ido, Y. Resistance to Obesity in SOD1 Deficient Mice with High-Fat/High-Sucrose Diet. Antioxidants 2022, 11, 1403. [Google Scholar]
- Mazzoli, A.; Crescenzo, R.; Cigliano, L.; Spagnuolo, M.S.; Cancelliere, R.; Gatto, C.; Iossa, S. Early Hepatic Oxidative Stress and Mitochondrial Changes Following Western Diet in Middle Aged Rats. Nutrients 2019, 11, 2670. [Google Scholar] [CrossRef]
- Mazzoli, A.; Spagnuolo, M.S.; Gatto, C.; Nazzaro, M.; Cancelliere, R.; Crescenzo, R.; Iossa, S.; Cigliano, L. Adipose Tissue and Brain Metabolic Responses to Western Diet-Is There a Similarity between the Two? Int. J. Mol. Sci. 2020, 21, 786. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Curcumin Attenuates Oxidative Stress and Activation of Redox-Sensitive Kinases in High Fructose- and High-Fat-Fed Male Wistar Rats. Sci. Pharm. 2015, 83, 159–175. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Paletas, K.; Pegiou, T.; Sarigianni, M.; Befani, C.; Koliakos, G. A Novel Assay for the Evaluation of the Prooxidant-Antioxidant Balance, before and after Antioxidant Vitamin Administration in Type II Diabetes Patients. Clin. Biochem. 2007, 40, 248–254. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sautin, Y.Y.; Oliver, W.J.; Roncal, C.; Mu, W.; Gabriela Sanchez-Lozada, L.; Rodriguez-Iturbe, B.; Nakagawa, T.; Benner, S.A. Lessons from Comparative Physiology: Could Uric Acid Represent a Physiologic Alarm Signal Gone Awry in Western Society? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2009, 179, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Hanley, A.; Casserly, L.F.; Cronin, C.J.; Abdalla, A.A.; Kiernan, T.J.; Murthy, B.V.R.; Hegarty, A.; Hannigan, A.; Nguyen, H.T. Independent and Conjoint Associations of Gout and Hyperuricaemia with Total and Cardiovascular Mortality. QJM 2013, 106, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Skrzep-Poloczek, B.; Poloczek, J.; Chełmecka, E.; Dulska, A.; Romuk, E.; Idzik, M.; Kazura, W.; Nabrdalik, K.; Gumprecht, J.; Jochem, J.; et al. The Oxidative Stress Markers in the Erythrocytes and Heart Muscle of Obese Rats: Relate to a High-Fat Diet but Not to DJOS Bariatric Surgery. Antioxidants 2020, 9, 183. [Google Scholar] [CrossRef]
- Francik, R.; Kryczyk, J.; Krośniak, M.; Berköz, M.; Sanocka, I.; Francik, S. The Neuroprotective Effect of Cornus MAS on Brain Tissue of Wistar Rats. Sci. World J. 2014, 2014, 847368. [Google Scholar] [CrossRef]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martínez-Pérez, M.M.; Calderón-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate Juice Increases Levels of Paraoxonase1 (PON1) Expression and Enzymatic Activity in Streptozotocin-Induced Diabetic Mice Fed with a High-Fat Diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate Juice Consumption Reduces Oxidative Stress, Atherogenic Modifications to LDL, and Platelet Aggregation: Studies in Humans and in Atherosclerotic Apolipoprotein E-Deficient Mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Grujić, V.R.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57. [Google Scholar] [CrossRef]
- Nankam, P.A.N.; Nguelefack, T.B.; Goedecke, J.H. Contribution of Adipose Tissue Oxidative Stress to Obesity-Associated Diabetes Risk and Ethnic Differences: Focus on Women of African Ancestry. Antioxidants 2021, 10, 622. [Google Scholar] [CrossRef]
- Celep, E.; Aydın, A.; Kırmızıbekmez, H.; Yesilada, E. Appraisal of in Vitro and in Vivo Antioxidant Activity Potential of Cornelian Cherry Leaves. Food Chem. Toxicol. 2013, 62, 448–455. [Google Scholar] [CrossRef]
- Es Haghi, M.; Dehghan, G.; Banihabib, N.; Zare, S.; Mikaili, P.; Panahi, F. Protective Effects of Cornus Mas Fruit Extract on Carbon Tetrachloride Induced Nephrotoxicity in Rats. Indian J. Nephrol. 2014, 24, 291–296. [Google Scholar] [CrossRef]
- Oczkowski, M.; Wilczak, J.; Dziendzikowska, K.; Øvrevik, J.; Myhre, O.; Lankoff, A.; Kruszewski, M.; Gromadzka-Ostrowska, J. Dietary Intervention with Blackcurrant Pomace Protects Rats from Testicular Oxidative Stress Induced by Exposition to Biodiesel Exhaust. Antioxidants 2022, 11, 1562. [Google Scholar] [CrossRef]
- Nisu, P.A.; Sa, P.; Kim, M. Nitric oxide products are not associated with metabolic syndrome. J. Med. Biochem. 2019, 38, 361–367. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, L.; Ouyang, X. Advanced Oxidation Protein Products Play Critical Roles in Liver Diseases. Eur. J. Clin. Investig. 2019, 49, e13098. [Google Scholar] [CrossRef]
- Grzebyk, E.; Piwowar, A. Oxidatively Modified Forms of Albumin in Patients with Risk Factors of Metabolic Syndrome. J. Endocrinol. Investig. 2014, 37, 819–827. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Marino, T.; Galano, A.; Russo, N. Radical Scavenging Ability of Gallic Acid toward OH and OOH Radicals. Reaction Mechanism and Rate Constants from the Density Functional Theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wójcicka, G.; Jamroz, A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis 2003, 170, 21–29. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Mondanelli, N.; Corallo, C.; Barbarino, M.; Giannotti, S.; Gallo, I.; Giordano, A.; Fioravanti, A. MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-κB Pathway in Human Osteoarthritic Chondrocytes. Cells 2019, 8, 874. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 13572. [Google Scholar] [CrossRef] [PubMed]
- Derdemezis, C.S.; Kiortsis, D.N.; Tsimihodimos, V.; Petraki, M.P.; Vezyraki, P.; Elisaf, M.S.; Tselepis, A.D. Effect of Plant Polyphenols on Adipokine Secretion from Human SGBS Adipocytes. Biochem Res Int. 2011, 2011, 285618. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castillejo, R.; Garcimart, A.; Hern, M. Proanthocyanidins: Impact on Gut Microbiota and Intestinal Action Mechanisms in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 5369. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]




| Total Contents | BC | CC |
| TPC (mg GAE/L) | 1392 ± 215 | 996 ± 95 |
| TF3C (mg B1E/L) | 1043 ± 60 | 187 ± 18 |
| TAC (mg Cy3GE/L) | 89 ± 10 | 51 ± 8 |
| Individual compounds | BC | CC |
| Tartaric acid (mg/L) | ND | 101.2 ± 8.7 |
| Malic acid (mg/L) | 472.2 ± 28.2 | 778.4 ± 47.5 |
| Citric acid (mg/L) | 1481 ± 169 | 50.8 ± 8.1 |
| Gallic acid (mg/L) | 1.1 ± 0.2 | 8.9 ± 1.9 |
| Protocatechuic acid (mg/L) | 0.3 ± 0.1 | 0.6 ± 0.1 |
| Epigallocatechin (mg/L) | 7.9 ± 0.1 | ND |
| Catechin (mg/L) | 6.0 ± 0.3 | ND |
| Epicatechin (mg/L) | 5.6 ± 0.3 | ND |
| Procyanidin B1 (mg/L) | 0.6 ± 0.1 | ND |
| Procyanidin B2 (mg/L) | 3.1 ± 0.4 | ND |
| Rutin (mg/L) | 0.30 ± 0.02 | 0.03 ± 0.01 |
| Parameter | Control Group | HFF | BC | CC | p |
|---|---|---|---|---|---|
| O2•− (μmol NBT/min/L) | 48 (37–51) | 51 (44–57) | 49 (46–55) | 41 (39–47) #† | 0.111 |
| PAB (U/L) | 118 (112–123) | 96 (93–109) * | 119 (112–126) # | 109 (99–115) | 0.029 |
| MDA (µmol/L) | 2.93 (2.59–3.19) | 2.74 (2.52–2.89) | 2.70 (2.44–3.04) | 3.41 (2.89–3.70) #† | 0.058 |
| AOPP (µmol/L) | 57.7 (53.6–61.2) | 54.4 (45.9–63.1) | 50.2 (46.4–53.9) ** | 49.9 (43.4–54.3) * | 0.052 |
| IMA (ABSU) | 0.050 (0.046–0.060) | 0.052 (0.046–0.057) | 0.048 (0.045–0.057) | 0.081 (0.039–0.301) | 0.664 |
| SOD (U/L) | 141 (139–144) | 141 (140–142) | 141 (139–142) | 142 (141–144) | 0.639 |
| SHG (mmol/L) | 0.216 (0.173–0.222) | 0.216 (0.190–0.262) | 0.199 (0.182–0.225) | 0.184 (0.148–0.277) | 0.803 |
| Parameter | Control Group | HFF | BC | CC | p |
|---|---|---|---|---|---|
| TOS (μmoL/L) | 772 (686–815) | 841 (794–890) * | 887 (863–1228) ** | 777 (750–854) † | 0.014 |
| O2•− (μmol NBT/min/L) | 2440 (2310–2580) | 2425 (2310–2760) | 2230 (2000–2310) *## | 2230 (2110–2440) | 0.042 |
| PAB (U/L) | 226 (206–246) | 234 (230–259) | 259 (244–265) * | 230 (209–237) † | 0.060 |
| MDA (µmol/L) | 93.3 (83.0–119) | 97.4 (88.2–120.7) | 79.3 (60.7–103.7) | 64.1 (54.1–81.8) *# | 0.065 |
| AOPP (µmol/L) | 1381 (1355–1475) | 1506 (1384–1604) | 1741 (1676–1869) **## | 1526 (1418–1680) † | 0.003 |
| IMA (ABSU) | 0.543 (539–595) | 0.622 (0.559–0.638) | 0.654 (0.551–0.681) | 0.591 (0.542–0.628) | 0.234 |
| PON1 (U/L) | 0 (0–0) | 85 (30–330) ** | 430 (360–490) ***## | 400 (340–410) ***# | <0.001 |
| TAS (μmoL/L) | 14,010 (13,780–14,220) | 11,345 (10,790–11,520) *** | 11,420 (11,230–12,240) *** | 13,160 (12,420–14,010) ###† | <0.001 |
| SOD (U/L) | 210 (0–240) | 1260 (1180–1320) *** | 860 (810–1290) *** | 1320 (1300–1345) *** | <0.001 |
| SHG (mmol/L) | 8.71 (8.38–9.89) | 8.60 (7.50–10.3) | 8.44 (8.09–9.06) | 8.27 (7.77–8.64) | 0.589 |
| TAS/TOS | 18.4 (16.2–20.4) | 13.2 (12.3–14.3) *** | 12.6 (9.3–13.6) ** | 17.9 (15.3–18.2) ##†† | <0.001 |
| Parameter | Control Group | HFF | BC | CC | p |
|---|---|---|---|---|---|
| TOS (μmoL/L) | 24.5 (22.0–27.5) | 38.0 (24.0–39.0) | 20.0 (14.5–20.0) ## | 16.0 (14.0–19.0) *### | 0.003 |
| O2•− (μmol NBT/min/L) | 160 (150–160) | 160 (150–160) | 160 (145–160) | 160 (150–160) | 0.954 |
| PAB (U/L) | 386 (373–392) | 380 (378–386) | 385 (381–397) | 388 (387–389) # | 0.213 |
| MDA (µmol/L) | 11.5 (8.89–15.6) | 13.7 (11.1–17.0) | 9.26 (8.52–10.4) # | 11.1 (7.04–12.6) | 0.110 |
| AOPP (µmoL/L) | 376 (347–392) | 319 (296–363) | 298 (283–343) | 319 (305–354) | 0.169 |
| IMA (ABSU) | 0.528 (0.497–0.538) | 0.526 (0.513–0.550) | 0.542 (0.504–0.545) | 0.486 (0.460–0.516) #† | 0.103 |
| PON1 (U/L) | 15 (0–30) | 10 (10–20) | 5 (0–10) # | 0 (0–0) *## | 0.010 |
| TAS (μmoL/L) | 1955 (1830–2030) | 2375 (2170–2450) * | 2025 (1790–2245) # | 2180 (2110–2180) | 0.027 |
| SOD (U/L) | 1275 (1250–1310) | 1225 (1210–1240) | 1245 (1235–1275) | 1240 (1200–1290) | 0.221 |
| SHG (mmoL/L) | 0.319 (0.174–0.450) | 0.334 (0.232–0.406) | 0.246 (0.210–0.276) | 0.218 (0.174–0.304) | 0.254 |
| TAS/TOS | 75.4 (72.4–91.8) | 58.6 (50.8–102.1) | 107 (85.4–135) | 132 (120–141) *## | 0.010 |
| Plasma | ||||
| Predictors | Dependent variable visceral fat %, F = 11.3, p < 0.001, R2 = 0.617, adjusted R2 = 0.563 | |||
| Unstandardized | Standardized Coefficients | |||
| B | Standard Error | β | p | |
| Experimental group | 0.821 | 0.243 | 0.486 | 0.003 |
| SOD (U/L) | 0.190 | 0.093 | 0.291 | 0.054 |
| PAB (U/L) | −0.044 | 0.017 | −0.348 | 0.020 |
| Liver | ||||
| Predictors | Dependent variable visceral fat %, F = 9.6, p < 0.001, R2 = 0.552, adjusted R2 = 0.523 | |||
| Unstandardized | Standardized Coefficients | |||
| B | Standard Error | β | p | |
| SOD (U/L) | 0.002 | 0.000 | 0.639 | <0.001 |
| O2•− (μmol NBT/min/L) | −0.002 | 0.001 | −0.225 | 0.087 |
| Adipose tissue | ||||
| Predictors | Dependent variable visceral fat %, F = 19.1, p < 0.001, R2 = 0.633, adjusted R2 = 0.567 | |||
| Unstandardized | Standardized Coefficients | |||
| B | Standard Error | β | p | |
| Experimental group | 1.291 | 0.212 | 0.818 | <0.001 |
| SOD (U/L) | −0.012 | 0.004 | −0.343 | 0.008 |
| AOPP (μmoL/L) | −0.002 | 0.001 | −0.258 | 0.036 |
| TOS (μmoL/L) | 0.060 | 0.020 | 0.422 | 0.006 |
| TAS (μmoL/L) | −0.002 | 0.001 | −0.327 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunovic, M.; Kotur-Stevuljevic, J.; Arsic, A.; Milosevic, M.; Todorovic, V.; Guzonjic, A.; Vucic, V.; Petrovic, S. Antioxidative Effects of Black Currant and Cornelian Cherry Juices in Different Tissues of an Experimental Model of Metabolic Syndrome in Rats. Antioxidants 2023, 12, 1148. https://doi.org/10.3390/antiox12061148
Paunovic M, Kotur-Stevuljevic J, Arsic A, Milosevic M, Todorovic V, Guzonjic A, Vucic V, Petrovic S. Antioxidative Effects of Black Currant and Cornelian Cherry Juices in Different Tissues of an Experimental Model of Metabolic Syndrome in Rats. Antioxidants. 2023; 12(6):1148. https://doi.org/10.3390/antiox12061148
Chicago/Turabian StylePaunovic, Marija, Jelena Kotur-Stevuljevic, Aleksandra Arsic, Maja Milosevic, Vanja Todorovic, Azra Guzonjic, Vesna Vucic, and Snjezana Petrovic. 2023. "Antioxidative Effects of Black Currant and Cornelian Cherry Juices in Different Tissues of an Experimental Model of Metabolic Syndrome in Rats" Antioxidants 12, no. 6: 1148. https://doi.org/10.3390/antiox12061148