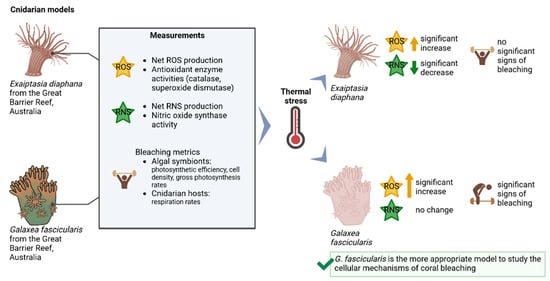
Comparing the Role of ROS and RNS in the Thermal Stress Response of Two Cnidarian Models, Exaiptasia diaphana and Galaxea fascicularis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of Symbiodiniacean Communities from Galaxea fascicularis Colonies
2.2. Experimental Setups and Sample Processing
2.3. Photosynthetic Performance and Respiration
2.4. Symbiodiniacean Cell Density, Sea Anemone Host Protein, and Coral Surface Area
2.5. ROS and RNS Quantification in Freshly Isolated Symbionidiaceae by Flow Cytometry
2.6. Enzyme Activities Involved in ROS Scavenging and RNS Synthesis
2.7. Data Analysis
3. Results
3.1. Bleaching Metrics of Exaiptasia diaphana and Galaxea fascicularis
3.2. Net ROS and RNS Production during Thermal Stress in Exaiptasia diaphana and Galaxea fascicularis
3.3. Activities of ROS-Scavenging and RNS-Synthesizing Enzymes during Thermal Stress in Exaiptasia diaphana and Galaxea fascicularis
4. Discussion
4.1. GBR-Sourced Exaiptasia diaphana Is Not a Suitable Model to Study Coral Bleaching
4.2. The Oxidative Stress Response during Thermal Stress Was More Marked in Galaxea fascicularis than in Exaiptasia diaphana and Aligned with the Extent of Physiological Stress
4.3. Ambiguous Role of RNS in the Thermal Stress Response of Exaiptasia diaphana and Galaxea fascicularis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Muscatine, L.; Cernichiari, E. Assimilation of Photosynthetic Products of Zooxanthellae by a Reef Coral. Biol. Bull. 1969, 137, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Grottoli, A.G.; Rodrigues, L.J.; Palardy, J.E. Heterotrophic Plasticity and Resilience in Bleached Corals. Nature 2006, 440, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Suggett, D.J.; Smith, D.J. Coral Bleaching Patterns Are the Outcome of Complex Biological and Environmental Networking. Glob. Chang. Biol. 2020, 26, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.A.; Davy, S.K. Cell Biology of Coral Bleaching. In Coral Bleaching; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 189–211. ISBN 9783319753935. [Google Scholar]
- Weis, V.M. Cellular Mechanisms of Cnidarian Bleaching: Stress Causes the Collapse of Symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.; Larkum, A.W.D.; Vass, I. A Review: The Role of Reactive Oxygen Species in Mass Coral Bleaching. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 459–482. [Google Scholar]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- Mehler, A.H. Studies on Reactions of Illuminated Chloroplasts. II. Stimulation and Inhibition of the Reaction with Molecular Oxygen. Arch. Biochem. Biophys. 1951, 34, 339–351. [Google Scholar] [CrossRef]
- Suggett, D.J.; Warner, M.E.; Smith, D.J.; Davey, P.; Hennige, S.; Baker, N.R. Photosynthesis and Production of Hydrogen Peroxide by Symbiodinium (Pyrrhophyta) Phylotypes with Different Thermal Tolerances. J. Phycol. 2008, 44, 948–956. [Google Scholar] [CrossRef]
- Asada, K.; Allen, J.; Foyer, C.H.; Matthijs, H.C.P. The Water-Water Cycle as Alternative Photon and Electron Sinks. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Active Oxygen in Photosynthesis. Photoinhibition 1987, 227–287. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Shick, J.M. Effects of Irradiance and Ultraviolet Radiation on Photoadaptation in the Zooxanthellae of Aiptasia pallida: Primary Production, Photoinhibition, and Enzymic Defenses against Oxygen Toxicity. Mar. Biol. 1989, 102, 243–255. [Google Scholar] [CrossRef]
- Merle, P.L.; Sabourault, C.; Richier, S.; Allemand, D.; Furla, P. Catalase Characterization and Implication in Bleaching of a Symbiotic Sea Anemone. Free Radic. Biol. Med. 2007, 42, 236–246. [Google Scholar] [CrossRef]
- Roberty, S.; Fransolet, D.; Cardol, P.; Plumier, J.C.; Franck, F. Imbalance between Oxygen Photoreduction and Antioxidant Capacities in Symbiodinium Cells Exposed to Combined Heat and High Light Stress. Coral Reefs 2015, 34, 1063–1073. [Google Scholar] [CrossRef]
- Matta, J.L.; Govind, N.S.; Trench, R.K. Polyclonal Antibodies against Iron-Superoxide Dismutase from Escherichia coli B Cross-React with Superoxide Dismutases from Symbiodinium microadriacticum (Dinophyceae). J. Phycol. 1992, 28, 343–346. [Google Scholar] [CrossRef]
- Shick, J.M.; Lesser, M.P.; Dunlap, W.C.; Stochaj, W.R.; Chalker, B.E.; Won, J.W. Depth-Dependent Responses to Solar Ultraviolet Radiation and Oxidative Stress in the Zooxanthellate Coral Acropora microphthalma. Mar. Biol. 1995, 122, 41–51. [Google Scholar] [CrossRef]
- Richier, S.; Merle, P.L.; Furla, P.; Pigozzi, D.; Sola, F.; Allemand, D. Characterization of Superoxide Dismutases in Anoxia- and Hyperoxia-Tolerant Symbiotic Cnidarians. Biochim. Biophys. Acta-Gen. Subj. 2003, 1621, 84–91. [Google Scholar] [CrossRef]
- Lesser, M.P.; Farrell, J.H. Exposure to Solar Radiation Increases Damage to Both Host Tissues and Algal Symbionts of Corals during Thermal Stress. Coral Reefs 2004, 23, 367–377. [Google Scholar] [CrossRef]
- Plantivaux, A.; Furla, P.; Zoccola, D.; Garello, G.; Forcioli, D.; Richier, S.; Merle, P.L.; Tambutté, É.; Tambutté, S.; Allemand, D. Molecular Characterization of Two CuZn-Superoxide Dismutases in a Sea Anemone. Free Radic. Biol. Med. 2004, 37, 1170–1181. [Google Scholar] [CrossRef]
- Lesser, M.P.; Shick, J.M. Photoadaption and Defenses against Oxygen Toxicity in Zooxanthellae from Natural Populations of Symbiotic Cnidarians. J. Exp. Mar. Bio. Ecol. 1989, 134, 129–141. [Google Scholar] [CrossRef]
- Krueger, T.; Hawkins, T.D.; Becker, S.; Pontasch, S.; Dove, S.; Hoegh-Guldberg, O.; Leggat, W.; Fisher, P.L.; Davy, S.K. Differential Coral Bleaching-Contrasting the Activity and Response of Enzymatic Antioxidants in Symbiotic Partners under Thermal Stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 190, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Elevated Temperatures and Ultraviolet Radiation Cause Oxidative Stress and Inhibit Photosynthesis in Symbiotic Dinoflagellates. Limnol. Oceanogr. 1996, 41, 271–283. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; De Vargas, C.; Yadav, S.N.; Milligant, A.J.; Häggblom, M.; Falkowski, P.G. Membrane Lipids of Symbiotic Algae Are Diagnostic of Sensitivity to Thermal Bleaching in Corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef]
- Smith, D.J.; Suggett, D.J.; Baker, N.R. Is Photoinhibition of Zooxanthellae Photosynthesis the Primary Cause of Thermal Bleaching in Corals? Glob. Chang. Biol. 2005, 11, 1–11. [Google Scholar] [CrossRef]
- Wooldridge, S.A. Breakdown of the Coral-Algae Symbiosis: Towards Formalising a Linkage between Warm-Water Bleaching Thresholds and the Growth Rate of the Intracellular Zooxanthellae. Biogeosciences 2013, 10, 1647–1658. [Google Scholar] [CrossRef]
- Wooldridge, S.A. A New Conceptual Model for the Warm-Water Breakdown of the Coralalgae Endosymbiosis. Mar. Freshw. Res. 2009, 60, 483–496. [Google Scholar] [CrossRef]
- Rädecker, N.; Pogoreutz, C.; Gegner, H.M.; Cárdenas, A.; Roth, F.; Bougoure, J.; Guagliardo, P.; Wild, C.; Pernice, M.; Raina, J.B.; et al. Heat Stress Destabilizes Symbiotic Nutrient Cycling in Corals. Proc. Natl. Acad. Sci. USA 2021, 118, e2022653118. [Google Scholar] [CrossRef]
- Baker, D.M.; Freeman, C.J.; Wong, J.C.Y.; Fogel, M.L.; Knowlton, N. Climate Change Promotes Parasitism in a Coral Symbiosis. ISME J. 2018, 12, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.E.; Postle, A.D.; Achterberg, E.P. Nutrient Enrichment Can Increase the Susceptibility of Reef Corals to Bleaching. Nat. Clim. Chang. 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Hawkins, T.D.; Bradley, B.J.; Davy, S.K. Nitric Oxide Mediates Coral Bleaching through an Apoptotic-like Cell Death Pathway: Evidence from a Model Sea Anemone-Dinoflagellate Symbiosis. FASEB J. 2013, 27, 4790–4798. [Google Scholar] [CrossRef]
- Hawkins, T.D.; Davy, S.K. Nitric Oxide Production and Tolerance Differ among Symbiodinium Types Exposed to Heat Stress. Plant Cell Physiol. 2012, 53, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Weis, V. Nitric Oxide and Cnidarian Bleaching: An Eviction Notice Mediates Breakdown of a Symbiosis. J. Exp. Biol. 2006, 209, 2804–2810. [Google Scholar] [CrossRef]
- Trapido-Rosenthal, H.; Zielke, S.; Owen, R.; Buxton, L.; Boeing, B.; Bhagooli, R.; Archer, J. Increased Zooxanthellae Nitric Oxide Synthase Activity Is Associated With Coral Bleaching. Biol. Bull. 2005, 208, 3–6. [Google Scholar] [CrossRef]
- Bouchard, J.N.; Yamasaki, H. Heat Stress Stimulates Nitric Oxide Production in Symbiodinium microadriaticum: A Possible Linkage between Nitric Oxide and the Coral Bleaching Phenomenon. Plant Cell Physiol. 2008, 49, 641–652. [Google Scholar] [CrossRef]
- Hawkins, T.D.; Davy, S.K. Nitric Oxide and Coral Bleaching: Is Peroxynitrite Generation Required for Symbiosis Collapse? J. Exp. Biol. 2013, 216, 3185–3188. [Google Scholar] [CrossRef]
- Hawkins, T.D.; Krueger, T.; Wilkinson, S.P.; Fisher, P.L.; Davy, S.K. Antioxidant Responses to Heat and Light Stress Differ with Habitat in a Common Reef Coral. Coral Reefs 2015, 34, 1229–1241. [Google Scholar] [CrossRef]
- Hawkins, T.D.; Krueger, T.; Becker, S.; Fisher, P.L.; Davy, S.K. Differential Nitric Oxide Synthesis and Host Apoptotic Events Correlate with Bleaching Susceptibility in Reef Corals. Coral Reefs 2014, 33, 141–153. [Google Scholar] [CrossRef]
- Jury, C.P.; Boeing, B.M.; Trapido-Rosenthal, H.; Gates, R.D.; Toonen, R.J. Nitric Oxide Production Rather than Oxidative Stress and Cell Death Is Associated with the Onset of Coral Bleaching in Pocillopora acuta. PeerJ 2022, 10, e13321. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.F. Exploring the Cellular Mechanisms of Cnidarian Bleaching in the Sea Anemone Aiptasia pallida. Doctoral Thesis, Oregon State University, Corvallis, OR, USA, 2007; pp. 1–134. [Google Scholar]
- Dungan, A.M.; Maire, J.; Perez-Gonzalez, A.; Blackall, L.L.; Van Oppen, M.J.H. Lack of Evidence for the Oxidative Stress Theory of Bleaching in the Sea Anemone, Exaiptasia diaphana, under Elevated Temperature. Coral Reefs 2022, 41, 1161–1172. [Google Scholar] [CrossRef]
- Dungan, A.M.; Hartman, L.M.; Blackall, L.L.; van Oppen, M.J.H. Exploring Microbiome Engineering as a Strategy for Improved Thermal Tolerance in Exaiptasia diaphana. J. Appl. Microbiol. 2022, 132, 2940–2956. [Google Scholar] [CrossRef]
- Puntin, G.; Craggs, J.; Hayden, R.; Engelhardt, K.; McIlroy, S.; Sweet, M.; Baker, D.M.; Ziegler, M. The Reef-Building Coral Galaxea fascicularis: A New Model System for Coral Symbiosis. Coral Reefs 2022, 42, 239–252. [Google Scholar] [CrossRef]
- Weis, V.M.; Davy, S.K.; Hoegh-Guldberg, O.; Rodriguez-Lanetty, M.; Pringle, J.R. Cell Biology in Model Systems as the Key to Understanding Corals. Trends Ecol. Evol. 2008, 23, 369–376. [Google Scholar] [CrossRef]
- Tortorelli, G.; Belderok, R.; Davy, S.K.; Mcfadden, G.I.; van Oppen, M.J.H.; Levy, O. Host Genotypic Effect on Algal Symbiosis Establishment in the Coral Model, the Anemone Exaiptasia diaphana, From the Great Barrier Reef. Front. Mar. Sci. 2020, 6, 833. [Google Scholar] [CrossRef]
- Hartman, L.M.; van Oppen, M.J.H.; Blackall, L.L. The Effect of Thermal Stress on the Bacterial Microbiome of Exaiptasia diaphana. Microorganisms 2020, 8, 20. [Google Scholar] [CrossRef]
- Hume, B.C.C.; D’Angelo, C.; Smith, E.G.; Stevens, J.R.; Burt, J.; Wiedenmann, J. Symbiodinium thermophilum sp. nov., a Thermotolerant Symbiotic Alga Prevalent in Corals of the World’s Hottest Sea, the Persian/Arabian Gulf. Sci. Rep. 2015, 5, 8562. [Google Scholar] [CrossRef]
- Hume, B.; D’Angelo, C.; Burt, J.; Baker, A.C.; Riegl, B.; Wiedenmann, J. Corals from the Persian/Arabian Gulf as Models for Thermotolerant Reef-Builders: Prevalence of Clade C3 Symbiodinium, Host Fluorescence and Ex Situ Temperature Tolerance. Mar. Pollut. Bull. 2013, 72, 313–322. [Google Scholar] [CrossRef]
- Hume, B.C.C.; Smith, E.G.; Ziegler, M.; Warrington, H.J.M.; Burt, J.A.; LaJeunesse, T.C.; Wiedenmann, J.; Voolstra, C.R. SymPortal: A Novel Analytical Framework and Platform for Coral Algal Symbiont next-Generation Sequencing ITS2 Profiling. Mol. Ecol. Resour. 2019, 19, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Dungan, A.M.; Hartman, L.; Tortorelli, G.; Belderok, R.; Lamb, A.M.; Pisan, L.; Mcfadden, G.I.; Blackall, L.L.; Van Oppen, M.J.H. Exaiptasia diaphana from the Great Barrier Reef: A Valuable Resource for Coral Symbiosis Research. Symbiosis 2020, 80, 196–206. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria,, 2022. [Google Scholar]
- Lavy, A.; Eyal, G.; Neal, B.; Keren, R.; Loya, Y.; Ilan, M. A Quick, Easy and Non-Intrusive Method for Underwater Volume and Surface Area Evaluation of Benthic Organisms by 3D Computer Modelling. Methods Ecol. Evol. 2015, 6, 521–531. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Mora, F.G.; Sousa, A.P.; Almeida-Santos, T.; Paiva, A.; João, R.-S. Fluorescent Probes for the Detection of Reactive Oxygen Species. Asian J. Androl. 2020, 22, 465–471. [Google Scholar]
- Mydlarz, L.D.; Palmer, C.V. The Presence of Multiple Phenoloxidases in Caribbean Reef-Building Corals. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2011, 159, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Gundlach, K.A.; Watson, G.M. The Effects of Symbiotic State and Nutrient Availability on the Cnidom in the Model Sea Anemone, Exaiptasia diaphana. Mar. Biol. 2019, 166, 31. [Google Scholar] [CrossRef]
- Sorek, M.; Schnytzer, Y.; Waldman Ben-Asher, H.; Caspi, V.C.; Chen, C.S.; Miller, D.J.; Levy, O. Setting the Pace: Host Rhythmic Behaviour and Gene Expression Patterns in the Facultatively Symbiotic Cnidarian Aiptasia Are Determined Largely by Symbiodinium. Microbiome 2018, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Klein, S.G.; Schmidt-Roach, S.; Campana, S.; Cziesielski, M.J.; Chen, J.E.; Duarte, C.M.; Aranda, M. Unfamiliar Partnerships Limit Cnidarian Holobiont Acclimation to Warming. Glob. Chang. Biol. 2020, 26, 5539–5553. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.L.; Sproles, A.E.; Oakley, C.A.; Grossman, A.R.; Weis, V.M.; Davy, S.K. Menthol-Induced Bleaching Rapidly and Effectively Provides Experimental Aposymbiotic Sea Anemones (Aiptasia sp.) for Symbiosis Investigations. J. Exp. Biol. 2016, 219, 306–310. [Google Scholar] [CrossRef]
- Imlay, J.A.; Fridovich, I. Assay of Metabolic Superoxide Production in Escherichia coli. J. Biol. Chem. 1991, 266, 6957–6965. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Mestdagh, M.M.; Rouxhet, P.G. Reversible Inhibition and Irreversible Inactivation of Catalase in Presence of Hydrogen Peroxide. Biochim. Biophys. Acta 1996, 1295, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Saragosti, E.; Tchernov, D.; Katsir, A.; Shaked, Y. Extracellular Production and Degradation of Superoxide in the Coral Stylophora pistillata and Cultured Symbiodinium. PLoS ONE 2010, 5, e12508. [Google Scholar] [CrossRef] [PubMed]
- Armoza-Zvuloni, R.; Shaked, Y. Release of Hydrogen Peroxide and Antioxidants by the Coral Stylophora pistillata to Its External Milieu. Biogeosciences 2014, 11, 4587–4598. [Google Scholar] [CrossRef]
- Armoza-Zvuloni, R.; Schneider, A.; Sher, D.; Shaked, Y. Rapid Hydrogen Peroxide Release from the Coral Stylophora pistillata during Feeding and in Response to Chemical and Physical Stimuli. Sci. Rep. 2016, 6, 21000. [Google Scholar] [CrossRef]
- Higuchi, T.; Fujimura, H.; Arakaki, T.; Oomori, T. Activities of Antioxidant Enzymes ( SOD and CAT ) in the Coral Galaxea fascicularis against Increased Hydrogen Peroxide Concentrations in Seawater. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2009; pp. 926–930. [Google Scholar]
- Roger, L.M.; Russo, J.A.; Jinkerson, R.E.; Giraldo, J.P.; Lewinski, N.A. Engineered Nanoceria Alleviates Thermally Induced Oxidative Stress in Free-Living Breviolum minutum (Symbiodiniaceae, Formerly Clade B). Front. Mar. Sci. 2022, 9, 960173. [Google Scholar] [CrossRef]
- Setter, R.O.; Franklin, E.C.; Mora, C. Co-Occurring Anthropogenic Stressors Reduce the Timeframe of Environmental Viability for the World’s Coral Reefs. PLoS Biol. 2022, 20, e3001821. [Google Scholar] [CrossRef]
- van Oppen, M.J.H.; Oliver, J.K.; Putnam, H.M.; Gates, R.D. Building Coral Reef Resilience through Assisted Evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef]
- Chan, W.Y.; Peplow, L.M.; van Oppen, M.J.H. Interspecific Gamete Compatibility and Hybrid Larval Fitness in Reef-Building Corals: Implications for Coral Reef Restoration. Sci. Rep. 2019, 9, 4757. [Google Scholar] [CrossRef]
- Chakravarti, L.J.; Beltran, V.H.; van Oppen, M.J.H. Rapid Thermal Adaptation in Photosymbionts of Reef-Building Corals. Glob. Chang. Biol. 2017, 23, 4675–4688. [Google Scholar] [CrossRef]
- Buerger, P.; Alvarez-Roa, C.; Coppin, C.W.; Pearce, S.L.; Chakravarti, L.J.; Oakeshott, J.G.; Edwards, O.R.; van Oppen, M.J.H. Heat-Evolved Microalgal Symbionts Increase Coral Bleaching Tolerance. Sci. Adv. 2020, 6, eaba2498. [Google Scholar] [CrossRef]
- Rosado, P.M.; Leite, D.C.A.; Duarte, G.A.S.; Chaloub, R.M.; Jospin, G.; Nunes da Rocha, U.; Saraiva, J.P.; Dini-Andreote, F.; Eisen, J.A.; Bourne, D.G.; et al. Marine Probiotics: Increasing Coral Resistance to Bleaching through Microbiome Manipulation. ISME J. 2019, 13, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.; Wall, M.; Putchim, L.; Rattanawongwan, T.; Schroeder, R.; Hentschel, U.; Roik, A. Towards Enhancing Coral Heat Tolerance: A “ Microbiome Transplantation ” Treatment Using Inoculations of Homogenized Coral Tissues. Microbiome 2021, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Santoro, E.P.; Borges, R.M.; Espinoza, J.L.; Freire, M.; Messias, C.S.; Villela, H.D.; Pereira, L.M.; Vilela, C.L.; Rosado, J.G.; Cardoso, P.M.; et al. Coral Microbiome Manipulation Elicits Metabolic and Genetic Restructuring to Mitigate Heat Stress and Evade Mortality. Sci. Adv. 2021, 7, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; van Oppen, M.J.H. A Role for Bacterial Experimental Evolution in Coral Bleaching Mitigation? Trends Microbiol. 2022, 30, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Dungan, A.M.; Bulach, D.; Lin, H.; van Oppen, M.J.H.; Blackall, L.L. Development of a Free Radical Scavenging Bacterial Consortium to Mitigate Oxidative Stress in Cnidarians. Microb. Biotechnol. 2021, 14, 2025–2040. [Google Scholar] [CrossRef]
- Lajeunesse, T.C.; Bhagooli, R.; Hidaka, M.; DeVantier, L.; Done, T.; Schmidt, G.; Fitt, W.; Hoegh-Guldberg, O. Closely Related Symbiodinium spp. Differ in Relative Dominance in Coral Reef Host Communities across Environmental, Latitudinal and Biogeographic Gradients. Mar. Ecol. Ser. 2004, 284, 147–161. [Google Scholar] [CrossRef]
- Bongaerts, P.; Sampayo, E.M.; Bridge, T.C.L.; Ridgway, T.; Vermeulen, F.; Englebert, N.; Webster, J.M.; Hoegh-Guldberg, O. Symbiodinium Diversity in Mesophotic Coral Communities on the Great Barrier Reef: A First Assessment. Mar. Ecol. Prog. Ser. 2011, 439, 117–126. [Google Scholar] [CrossRef]
- Veron, J.E.N.; Stafford-Smith, M.G.; Turak, E.; DeVantier, L.M. Corals of the World. Available online: http://www.coralsoftheworld.org/species_factsheets/species_factsheet_summary/galaxea-fascicularis/?version=0.01 (accessed on 15 January 2023).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doering, T.; Maire, J.; Chan, W.Y.; Perez-Gonzalez, A.; Meyers, L.; Sakamoto, R.; Buthgamuwa, I.; Blackall, L.L.; van Oppen, M.J.H. Comparing the Role of ROS and RNS in the Thermal Stress Response of Two Cnidarian Models, Exaiptasia diaphana and Galaxea fascicularis. Antioxidants 2023, 12, 1057. https://doi.org/10.3390/antiox12051057
Doering T, Maire J, Chan WY, Perez-Gonzalez A, Meyers L, Sakamoto R, Buthgamuwa I, Blackall LL, van Oppen MJH. Comparing the Role of ROS and RNS in the Thermal Stress Response of Two Cnidarian Models, Exaiptasia diaphana and Galaxea fascicularis. Antioxidants. 2023; 12(5):1057. https://doi.org/10.3390/antiox12051057
Chicago/Turabian StyleDoering, Talisa, Justin Maire, Wing Yan Chan, Alexis Perez-Gonzalez, Luka Meyers, Rumi Sakamoto, Isini Buthgamuwa, Linda L. Blackall, and Madeleine J. H. van Oppen. 2023. "Comparing the Role of ROS and RNS in the Thermal Stress Response of Two Cnidarian Models, Exaiptasia diaphana and Galaxea fascicularis" Antioxidants 12, no. 5: 1057. https://doi.org/10.3390/antiox12051057