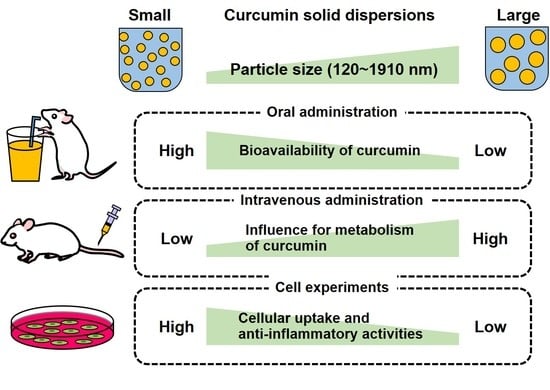
Effects of Particle Size of Curcumin Solid Dispersions on Bioavailability and Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of C-SDs
2.3. Characterization of C-SDs I-IV
2.4. Bioavailability of C-SDs after Oral Administration in Rats
2.5. Blood Profile of C-SDs after Intravenous Administration in Rats
2.6. Cellular Uptake and Anti-Inflammatory Activity of C-SDs
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of C-SDs
3.2. Oral Bioavailability of C-SDs
3.3. Blood Profile of C-SDs after Intravenous Administration
3.4. Cellular Uptake and Anti-Inflammatory Activities of C-SDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kim, S.H.; Thomas, M.J.; Paul, L.; Zingg, J.M.; Dolnikowski, G.G.; Roberts, S.B.; Kimura, F.; Miyazawa, T.; et al. Curcumin and piperine supplementation of obese mice under caloric restriction modulates body fat and interleukin-1β. Nutr. Metab. 2018, 15, 12. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Itaya, M.; Miyazawa, T.; Zingg, J.M.; Eitsuka, T.; Azzi, A.; Meydani, M.; Miyazawa, T.; Nakagawa, K. The differential cellular uptake of curcuminoids in vitro depends dominantly on albumin interaction. Phytomedicine 2019, 59, 152902. [Google Scholar] [CrossRef]
- Mimica, B.; Bucevic Popovic, V.; Banjari, I.; Jelicic Kadic, A.; Puljak, L. Methods Used for Enhancing the Bioavailability of Oral Curcumin in Randomized Controlled Trials: A Meta-Research Study. Pharmaceuticals 2022, 15, 939. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Kim, J.A.; Joung, H.J.; Ko, J.A.; Park, H.J. Preparation and characterization of curcumin solid dispersion using HPMC. J. Food Sci. 2020, 85, 3866–3873. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Mendonça, L.M.; Bergamaschi, M.M.; Queiroz, R.H.; Souza, G.E.; Antunes, L.M.; Freitas, L.A. Microparticles Containing Curcumin Solid Dispersion: Stability, Bioavailability and Anti-Inflammatory Activity. AAPS PharmSciTech. 2016, 17, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Cristina, C.; Raluca-Elena, Ș.; Georgiana, D.; Adriana, A.; Alina, M.H.; Ovidiu-Cristian, O.; Bogdan, S.V.; Ionela, A.N.; Bianca, T. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057. [Google Scholar]
- Harigae, T.; Nakagawa, K.; Miyazawa, T.; Inoue, N.; Kimura, F.; Ikeda, I.; Miyazawa, T. Metabolic fate of poly-(lactic-co-glycolic acid)-based curcumin nanoparticles following oral administration. Int. J. Nanomed. 2016, 11, 3009–3022. [Google Scholar]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Song, I.S.; Cha, J.S.; Choi, M.K. Characterization, In Vivo and In Vitro Evaluation of Solid Dispersion of Curcumin Containing d-α-Tocopheryl Polyethylene Glycol 1000 Succinate and Mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of the nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 9, 3937–3999. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q₁₀ as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733–5744. [Google Scholar]
- Ye, J.Y.; Chen, Z.Y.; Huang, C.L.; Huang, B.; Zheng, Y.R.; Zhang, Y.F.; Lu, B.Y.; He, L.; Liu, C.S.; Long, X.Y. A Non-Lipolysis Nanoemulsion Improved Oral Bioavailability by Reducing the First-Pass Metabolism of Raloxifene, and Related Absorption Mechanisms Being Studied. Int. J. Nanomed. 2020, 15, 6503–6518. [Google Scholar] [CrossRef]
- Kutscher, H.L.; Chao, P.; Deshmukh, M.; Singh, Y.; Hu, P.; Joseph, L.B.; Reimer, D.C.; Stein, S.; Laskin, D.L.; Sinko, P.J. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J. Control Release 2010, 143, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Aditya, N.P.; Yadira, G.E.; Ian, T.N. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, Y.; Junya, I.; Naoki, S.; Takumi, T.; Chikara, K.; Isabella, S.P.; Mirinthorn, J.; Katsuyuki, I.; Kiyotaka, N. Structural Analysis and Anti-Inflammatory Effect of a Digalactosyldiacylglycerol-Monoestolide, a Characteristic Glycolipid in Oats. Nutrients 2022, 14, 4153. [Google Scholar]
- Itaya, M.; Miyazawa, T.; Khalifa, S.; Shimizu, N.; Nakagawa, K. The inhibition of interaction with serum albumin enhances the physiological activity of curcumin by increasing its cellular uptake. Food Funct. 2022, 13, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Kanada, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, A.; Giovanna, A.; Vasileios, B.; Maria, L.B.; Georges, B.; Andrew, C.; Pier, S.C.; Gerhard, F.; Jürgen, G.; Boris, K.; et al. Safety and efficacy of lecithins (Lipidol) for all animal species. EFSA J. 2016, 14, 8. [Google Scholar]
- Peter, A.; Fernando, A.; Riccardo, C.; Birgit, D.; Metka, F.; Maria, J.F.; Pierre, G.; David, G.; Ursula, G.; Gunter, G.K.; et al. Re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2017, 15, 12. [Google Scholar]
- Francesco, D.; Yuwen, W.; Ji, L.; Qingrong, H. Preparation of Curcumin Sub-micrometer Dispersions by High-Pressure Homogenization. J. Agric. Food Chem. 2010, 58, 2848–2853. [Google Scholar]
- Chao, B.; Xiao, Q.M.; Sing, F.C.; Wen, J.W.; Ru, Y.; Liao, Y.H.; Albert, H.C.; Ying, Z. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine 2017, 13, 943–953. [Google Scholar]
- Praharsh, K.M.R.; Karthik, G.; Bala, S.S.N.; Jawahar, N.; Nirmala, P. Design, formulation, and evaluation of curcumin-loaded nanosponges for the effective management of colitis. J. Appl. Pharm. Sci. 2022, 12, 12. [Google Scholar]
- Qiumin, M.; Michael, D.P.; Qixin, Z. Nanoemulsions of thymol and eugenol co-emulsified by lauric arginate and lecithin. Food Chem. 2016, 206, 167–173. [Google Scholar]
- Hujun, X.; Fangfang, N.; Chengzhi, L.; Jieyu, S.; Gerui, R.; Zunyi, W.; Zhijun, S. Characterization and stability of peppermint oil emulsions using polyglycerol esters of fatty acids and milk proteins as emulsifiers. J. Food Sci. 2021, 86, 5148–5158. [Google Scholar]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Manisha, P.D.; Vinod, L.; Gordon, L.A.; Robert, J.L. Gastrointestinal Uptake of Biodegradable Microparticles: Effect of Particle Size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar]
- Michael, J.M.; Margaret, M.B.; Rebecca, M.H.; Marissa, E.W.; Nicholas, A.P.; Robert, L. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Darío, M.; Valentín, C. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar]









| Gene | Genbank ID | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| Il-1β | NM_008361.4 | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
| Cox-2 | NM_011198.5 | TCTGGTGCCTGGTCTGATGATGT | AGTCTGCTGGTTTGGAATAGTTGCT |
| Il-6 | X54542.1 | ACACATGTTCTCTGGGAAATCGT | AAGTGCATCATCGTTGTTCATACA |
| Mcp-1 | NM_011333.3 | AGGTCCCTGTCATGCTTCTGG | CTGCTGCTGGTGATCCTCTTG |
| Inos | M87039.1 | GGAATCTTGGAGCGAGTTGTGGA | GTGAGGGCTTGGCTGAGTGAG |
| Tnf-α | X02611.1 | GAAAGCATGATCCGCGACGT | CGAAGTTCAGTAGACAGAAG |
| Nf-κb | XM_021152061.2 | TGAAGAAGCGAGACCTGGAGCAA | GCACTGTCACCTGGAAGCAGAG |
| Tgf-β | NM_011577.2 | AGACATTCGGGAAGCAGTGC | AAAGACAGCCACTCAGGCGT |
| Ho-1 | NM_010442.2 | AGACCGCCTTCCTGCTCAAC | ACGAAGTGACGCCATCTGTGA |
| Cat | NM_009804.2 | AGCCAGAAGAGAAACCCACAGACT | AAGCCTTCCGCCTCTCCAACA |
| Nrf-2 | NM_010902.5 | CTTCCATTTACGGAGACCCA | ATTCACGCATAGGAGCACTG |
| Gapdh | BC023196.2 | CATGTTCCAGTATGACTCCACTC | GGCCTCACCCCATTTGATGT |
| Classification | C-SDs I | C-SDs II | C-SDs III | C-SDs IV |
|---|---|---|---|---|
| Mean of particle diameters (nm) (Measured value (nm)) | 122 (123, 121) | 447 (448, 445) | 987 (991, 983) | 1910 (1903, 1914) |
| Zeta potential (mV) | −33.4 ± 1.9 | −33.5 ± 3.3 | −38.1 ± 3.1 | −26.6 ± 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, C.; Itaya-Takahashi, M.; Miyazawa, T.; Ito, J.; Parida, I.S.; Yamada, H.; Abe, A.; Shibata, M.; Someya, K.; Nakagawa, K. Effects of Particle Size of Curcumin Solid Dispersions on Bioavailability and Anti-Inflammatory Activities. Antioxidants 2023, 12, 724. https://doi.org/10.3390/antiox12030724
Kato C, Itaya-Takahashi M, Miyazawa T, Ito J, Parida IS, Yamada H, Abe A, Shibata M, Someya K, Nakagawa K. Effects of Particle Size of Curcumin Solid Dispersions on Bioavailability and Anti-Inflammatory Activities. Antioxidants. 2023; 12(3):724. https://doi.org/10.3390/antiox12030724
Chicago/Turabian StyleKato, Chihiro, Mayuko Itaya-Takahashi, Taiki Miyazawa, Junya Ito, Isabella Supardi Parida, Hiroki Yamada, Akari Abe, Mika Shibata, Keita Someya, and Kiyotaka Nakagawa. 2023. "Effects of Particle Size of Curcumin Solid Dispersions on Bioavailability and Anti-Inflammatory Activities" Antioxidants 12, no. 3: 724. https://doi.org/10.3390/antiox12030724