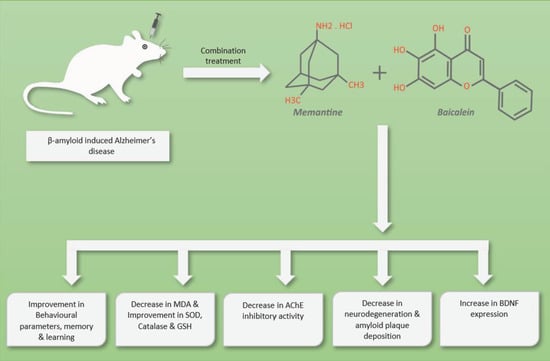
The Combination of Baicalein and Memantine Reduces Oxidative Stress and Protects against β-amyloid-Induced Alzheimer’s Disease in Rat Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Drugs and Chemicals
2.2. Experimental Animals
2.3. Experimental Design
2.4. Surgical Procedures
2.5. Behavioural Assessment
2.5.1. Locomotor Activity
2.5.2. Morris Water Maze (MWM) Test
2.5.3. Elevated Plus Maze (EPM) Test
2.5.4. Passive Avoidance (PA) Test
2.6. Biochemical Assessment
2.6.1. Measurement of Oxidative Stress Parameters
2.6.2. Measurement of Acetyl Cholinesterase Activity
2.7. Histopathological Assessment
2.8. Immunohistochemistry Assessment (IHC)
2.9. Statistical Analysis
3. Results
3.1. Behavioural Assessment
3.1.1. Locomotor Activity
3.1.2. Morris Water Maze Test
3.1.3. Elevated Plus Maze Test
3.1.4. Passive Avoidance Test
3.2. Effect of Oxidative Stress Parameters
3.3. Acetyl Cholinesterase Activity
3.4. Histopathological Study
3.4.1. Haematoxylin and Eosin Staining
3.4.2. Congo Red Staining
3.5. Immunohistochemistry Assessment (IHC)
3.5.1. Immunohistochemistry Assessment for β-amyloid
3.5.2. Immunohistochemistry Assessment for BDNF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. World Population Ageing 2019; United Nations: New York, NY, USA, 2019; ISBN 9789211483260. [Google Scholar]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and Regional Projections of the Economic Burden of Alzheimer’s Disease and Related Dementias from 2019 to 2050: A Value of Statistical Life Approach. EClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD Directly Links Aß to Mitochondrial Toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative Stress and Lipid Peroxidation Are Upstream of Amyloid Pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease and Its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Memantine: Pharmacologial Properties and Clinical Uses. Neurol. India 2004, 52, 307–309. [Google Scholar]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments for Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H. An Ethnopharmacological Review on the Therapeutical Properties of Flavonoids and Their Mechanisms of Actions: A Comprehensive Review Based on up to Date Knowledge. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An Overview of Pharmacological Activities of Baicalin and Its Aglycone Baicalein: New Insights into Molecular Mechanisms and Signaling Pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Cathcart, M.C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-Cancer Effects of Baicalein in Non-Small Cell Lung Cancer in-Vitro and in-Vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Hu, X.; Pan, J.; Zhang, G. Inhibitory Mechanism of Baicalein on Acetylcholinesterase: Inhibitory Interaction, Conformational Change, and Computational Simulation. Foods 2022, 11, 168. [Google Scholar] [CrossRef]
- Carradori, S.; Secci, D.; Petzer, J.P. MAO Inhibitors and Their Wider Applications: A Patent Review. Expert Opin. Ther. Pat. 2018, 28, 211–226. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Trifan, A.; Hancianu, M. Inhalation of Coriander Volatile Oil Increased Anxiolytic-Antidepressant-like Behaviors and Decreased Oxidative Status in Beta-Amyloid (1-42) Rat Model of Alzheimer’s Disease. Physiol. Behav. 2014, 131, 68–74. [Google Scholar] [CrossRef]
- Hruska, Z.; Dohanich, G.P. The Effects of Chronic Estradiol Treatment on Working Memory Deficits Induced by Combined Infusion of β-Amyloid (1-42) and Ibotenic Acid. Horm. Behav. 2007, 52, 297–306. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, S.; Shan, Y.L.; Wang, Y.G.; Cai, W.; Huang, X.H.; Liao, X.Y.; Li, H.Y.; Zhang, L.; Zhang, B.J.; et al. Baicalein Improves Behavioral Dysfunction Induced by Alzheimer’s Disease in Rats. Neuropsychiatr. Dis. Treat. 2016, 12, 3145–3152. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Trigonelline Protects Hippocampus against Intracerebral Aβ(1–40) as a Model of Alzheimer’s Disease in the Rat: Insights into Underlying Mechanisms. Metab. Brain Dis. 2019, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh Jajin, E.; Esmaeili, A.; Rahgozar, S.; Noorbakhshnia, M. Quercetin-Conjugated Superparamagnetic Iron Oxide Nanoparticles Protect AlCl3-Induced Neurotoxicity in a Rat Model of Alzheimer’s Disease via Antioxidant Genes, APP Gene, and MiRNA-101. Front. Neurosci. 2021, 14, 598617. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The Use of the Elevated. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.-K.; Chou, M.-K.; Shen, Y.-C.; Wang, Y.-H.; Yen, J.-C.; Chen, C.-F.; Lin, S.-K.; Liao, J.-F. Preventive Effect of Baicalein on Methamphetamine-Induced Amnesia in the Passive Avoidance Test in Mice. Pharmacology 2014, 93, 278–285. [Google Scholar] [CrossRef]
- Prema, A.; Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M.; Akbar, M.; Akbar, M.D. Fenugreek Seed Powder Nullified Aluminium Chloride Induced Memory Loss, Biochemical Changes, Aβ Burden and Apoptosis via Regulating Akt/GSK3β Signaling Pathway. PLoS ONE 2016, 11, e0165955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Paoletti, F.; Aldinucci, D.; Mocali, A.; Caparrini, A. A Sensitive Spectrophotometric Method for the Determination of Superoxide Dismutase Activity in Tissue Extracts. Anal. Biochem. 1986, 154, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Luck, H. Catalases. Section C: Methods for Determination of Enzyme Activity. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1971; pp. 885–888. [Google Scholar] [CrossRef]
- ELLMAN, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.P. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s Disease Neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Kasza, Á.; Penke, B.; Frank, Z.; Bozsó, Z.; Szegedi, V.; Hunya, Á.; Németh, K.; Kozma, G.; Fülöp, L. Studies for Improving a Rat Model of Alzheimer’s Disease: Icv Administration of Well-Characterized β-Amyloid 1-42 Oligomers Induce Dysfunction in Spatial Memory. Molecules 2017, 22, 2007. [Google Scholar] [CrossRef] [Green Version]
- Dudi, R.; Mehan, S. Neuroprotection of Brain Permeable Forskolin Ameliorates Behavioral, Biochemical and Histopathological Alterations in Rat Model of Intracerebral Hemorrhage. Pharmaspire 2018, 10, 68–86. [Google Scholar]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Khan, S.; Shad, K.F. Neuroprotective Effects of Curcumin and Vitamin D3 on Scopolamine-Induced Learning-Impaired Rat Model of Alzheimer’s Disease. In Neurological and Mental Disorders; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-83962-973-0. [Google Scholar]
- Pathak, D.; Shields, L.Y.; Mendelsohn, B.A.; Haddad, D.; Lin, W.; Gerencser, A.A.; Kim, H.; Brand, M.D.; Edwards, R.H.; Nakamura, K. The Role of Mitochondrially Derived ATP in Synaptic Vesicle Recycling. J. Biol. Chem. 2015, 290, 22325–22336. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of Amyloid β-Peptide-Associated Oxidative Stress and Brain Protein Modifications in the Pathogenesis of Alzheimer’s Disease and Mild Cognitive Impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Hemmelgarn, B.T.; Chuang, C.-C.; Best, T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-β Production in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 604658. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as Acetylcholinesterase Inhibitors: Current Therapeutic Standing and Future Prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Balkis, A.; Tran, K.; Lee, Y.Z.; Ng, K. Screening Flavonoids for Inhibition of Acetylcholinesterase Identified Baicalein as the Most Potent Inhibitor. J. Agric. Sci. 2015, 7, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a Potential Inhibitor against BACE1 and AChE: Mechanistic Comprehension through In Vitro and Computational Approaches. Nutrients 2019, 11, 2694. [Google Scholar] [CrossRef] [Green Version]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]













| Groups | MDA (nmol/mg of Protein) | SOD (µmol/mg of Protein) | CAT (nmol of H2O2 Decomposed/ min/mg of Protein) | GSH (µmol/mg of Protein) |
|---|---|---|---|---|
| Normal control | 4.10 ± 0.31 | 8.65 ± 0.48 | 9.88 ± 0.46 | 11.00 ± 0.36 |
| Disease control | 8.01 ± 0.63 ### | 4.84 ± 0.28 ### | 4.03 ± 0.60 ### | 4.95 ± 0.36 ### |
| Memantine (20 mg/kg) | 6.85 ± 0.30 | 6.09 ± 0.41 | 4.97 ± 0.36 | 5.95 ± 0.57 |
| Baicalein (10 mg/kg) | 6.22 ± 0.37 * | 6.63 ± 0.53 | 6.25 ± 0.52 * | 6.84 ± 0.58 * |
| Memantine (20 mg/kg) + Baicalein (5 mg/kg) | 5.84 ± 0.42 ** | 7.09 ± 0.57 * | 7.40 ± 0.51 ***/@ | 8.75 ± 0.37 ***/@@@ |
| Memantine (20 mg/kg) + Baicalein (10 mg/kg) | 4.96 ± 0.36 ***/@ | 7.88 ± 0.69 ***/@@ | 8.73 ± 0.62 ***/@@@ | 9.76 ± 0.41 ***/@@@ |
| Groups | MDA (nmol/mg of Protein) | SOD (µmol/mg of Protein) | CAT (nmol of H2O2 Decomposed/ min/mg of Protein) | GSH (µmol/mg of Protein) |
|---|---|---|---|---|
| Normal control | 4.16 ± 0.35 | 8.10 ± 0.46 | 8.71 ± 0.41 | 10.31 ± 0.67 |
| Disease control | 7.49 ± 0.43 ### | 4.41 ± 0.28 ### | 4.35 ± 0.34 ### | 5.06 ± 0.39 ### |
| Memantine (20 mg/kg) | 6.95 ± 0.42 | 5.25 ± 0.37 | 5.52 ± 0.30 | 6.36 ± 0.57 |
| Baicalein (10 mg/kg) | 5.37 ± 0.54 **/@ | 6.96 ± 0.29 ***/@ | 6.13 ± 0.29 ** | 7.20 ± 0.54 * |
| Memantine (20 mg/kg) + Baicalein (5 mg/kg) | 4.91 ± 0.33 ***/@@ | 7.46 ± 0.45 ***/@@ | 7.32 ± 0.45 ***/@@ | 8.02 ± 0.53 ** |
| Memantine (20 mg/kg) + Baicalein (10 mg/kg) | 4.55 ± 0.38 ***/@@ | 7.74 ± 0.48 ***/@@@ | 8.29 ± 0.43 ***/@@@ | 9.11 ± 0.45 ***/@ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, R.; Kulkarni, Y.A. The Combination of Baicalein and Memantine Reduces Oxidative Stress and Protects against β-amyloid-Induced Alzheimer’s Disease in Rat Model. Antioxidants 2023, 12, 707. https://doi.org/10.3390/antiox12030707
Jadhav R, Kulkarni YA. The Combination of Baicalein and Memantine Reduces Oxidative Stress and Protects against β-amyloid-Induced Alzheimer’s Disease in Rat Model. Antioxidants. 2023; 12(3):707. https://doi.org/10.3390/antiox12030707
Chicago/Turabian StyleJadhav, Ratnakar, and Yogesh A. Kulkarni. 2023. "The Combination of Baicalein and Memantine Reduces Oxidative Stress and Protects against β-amyloid-Induced Alzheimer’s Disease in Rat Model" Antioxidants 12, no. 3: 707. https://doi.org/10.3390/antiox12030707