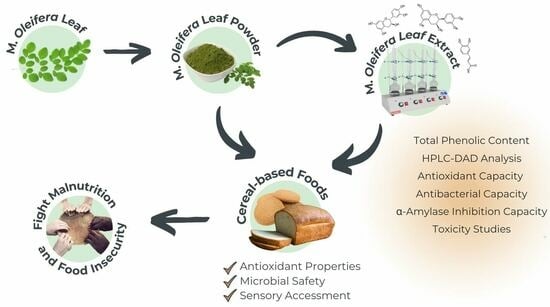
Elevating Cereal-Based Nutrition: Moringa oleifera Supplemented Bread and Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Extraction of Bioactive Compounds from Moringa oleifera
2.3. Characterisation of Moringa oleifera Extract
2.3.1. Total Phenolic Content and Antioxidant Capacity
2.3.2. Antibacterial Capacity
2.3.3. α-Amylase Inhibition
2.3.4. Phenolic Compounds Quantification
2.3.5. Cytotoxicity
2.4. Incorporation of Moringa oleifera Leaf Extract in Bread and Biscuits
2.4.1. Production of Breads and Biscuits
2.4.2. Antioxidant Capacity
2.4.3. Microbial Analysis
2.4.4. Sensory Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Moringa oleifera Extract
3.2. Characterisation of Fortified Breads and Biscuits
3.2.1. Physical Characterisation
3.2.2. Total Phenolic Content and Antioxidant Capacity
3.2.3. Microbial Contamination
3.2.4. Sensory Assessment of the Breads and Biscuits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar]
- Paraschivu, M.; Cotuna, O.; Matei, G.; Sarateanu, V. Are Food Waste and Food Loss a Real Threat for FOOD Security? Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2022, 22, 479–484. [Google Scholar]
- Hodas, F.; Zorzenon, M.R.T.; Milani, P.G. Moringa oleifera potential as a functional food and a natural food additive: A biochemical approach. An. Acad. Bras. Ciênc. 2021, 93, e20210571. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abdelwareth, A.; Sallam, I.E.; El Shorbagi, M.; Jehmlich, N.; Fritz-Wallace, K.; Schäpe, S.S.; Rolle-Kampczyk, U.; Ehrlich, A.; Wessjohann, L.A.; et al. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J. Adv. Res. 2020, 23, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martinez, E.J.; Gordo-Moreno, A.I.; Fernandez-de Cordova, M.L.; Ruiz-Medina, A. Preliminary Phytochemical Screening and Antioxidant Activity of Commercial Moringa oleifera Food Supplements. Antioxidants 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Hisam, E.E.A.; Rofiee, M.S.; Khalid, A.M.; Jalaluddin, A.F.; Yusof, M.I.M.; Idris, M.H.; Ramli, S.; James, R.J.; Yoeng, W.J.; Kek, T.L.; et al. Combined extract of Moringa oleifera and Centella asiatica modulates oxidative stress and senescence in hydrogen peroxide-induced human dermal fibroblasts. Turk. J. Biol. 2018, 42, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, A.R.M.H.A.; Navaratne, S.B.; Uthpala, T.G.G. Moringa oleifera Plant and the Nutritional and Medicinal Properties of Moringa olifera Leaves. In Trends & Prospects in Processing of Horticultural Crops; Chakraborty, I., Paul, P.K., Mani, A., Tiwary, A.K., Prasad, K., Eds.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2019; pp. 251–268. [Google Scholar]
- Oyeyinka, A.T.; Oyeyinka, S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018, 17, 127–136. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Verma, A.K.; Swarup, D. Moringa oleiferia leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. Food Sci. Technol. 2012, 47, 585–591. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Vonghirundecha, P.; Chusri, S.; Meunprasertdee, P.; Kaewmanee, T. Microencapsulated functional ingredients from a Moringa oleifera leaf polyphenol-rich extract: Characterization, antioxidant properties, in vitro simulated digestion, and storage stability. LWT 2022, 154, 112820. [Google Scholar] [CrossRef]
- Singh, D.; Arya, P.V.; Aggarwal, V.P.; Gupta, R.S. Evaluation of Antioxidant and Hepatoprotective Activities of Moringa oleifera Lam. Leaves in Carbon Tetrachloride-Intoxicated Rats. Antioxidants 2014, 3, 569–591. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crops Prod. 2013, 49, 419–421. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Karray, A.; Alonazi, M.; Jallouli, R.; Alanazi, H.; Ben Bacha, A. A Proteinaceous Alpha-Amylase Inhibitor from Moringa oleifera Leaf Extract: Purification, Characterization, and Insecticide Effects against C. maculates Insect Larvae. Molecules 2022, 27, 4222. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; ALAM, S.S.; Fatima, M.; Altaf, I.; Khan, F.; Afzal, A. Comparative Anti-Influenza Potential of Moringa oleifera Leaves and Amantadine Invitro. Pak. Postgrad. Med. J. 2017, 28, 127–131. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: An updated review. Nutr. Food Sci. 2020, 51, 255–277. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Kakde, S.B.; Masih, D.; Sonkar, C. Utilization of Moringa leaves powder as valuable food ingredients in pasta preparation. J. Pharmacogn. Phytochem. 2018, 7, 1053–1056. [Google Scholar]
- Khan, M.A.; Shakoor, S.; Ameer, K.; Farooqi, M.A.; Rohi, M.; Saeed, M.; Asghar, M.T.; Irshad, M.B.; Waseem, M.; Tanweer, S.; et al. Effects of Dehydrated Moringa (Moringa oleifera) Leaf Powder Supplementation on Physicochemical, Antioxidant, Mineral, and Sensory Properties of Whole Wheat Flour Leavened Bread. J. Food Qual. 2023, 2023, 4473000. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Evaluation of physical, sensorial, and antioxidant properties of gluten-free bread enriched with Moringa oleifera leaf powder. Eur. Food Res. Technol. 2018, 244, 189–195. [Google Scholar] [CrossRef]
- Fapetu, A.P.; Karigidi, K.O.; Akintimehin, E.S.; Olawuwo, T.; Adetuyi, F.O. Effect of partial substitution of wheat flour with Moringa oleifera leaf powder on physical, nutritional, antioxidant and antidiabetic properties of cookies. Bull. Natl. Res. Cent. 2022, 46, 46–53. [Google Scholar] [CrossRef]
- Dachana, K.B.; Rajiv, J.; Indrani, D.; Prakash, J. Effect of Dried Moringa (Moringa oleifera Lam) Leaves on Rheological, Microstructural, Nutritional, Textural and Organoleptic Characteristics of Cookies. J. Food Qual. 2010, 33, 660–677. [Google Scholar] [CrossRef]
- Gomes, S.M.; Albuquerque, D.; Santos, L. Innovative Approaches for Food: Using Natural Phenolic-Rich Extracts to Produce Value-Added Fresh Pasta. Int. J. Mol. Sci. 2023, 24, 12451. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. Incorporation of phenolic extracts from different by-products in yoghurts to create fortified and sustainable foods. Food Biosci. 2023, 51, 102293. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Gomes, S.M.; Miranda, R.; Santos, L. Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream. Sustainability 2023, 15, 14059. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Santana, C.M.; Ferrera, Z.S.; Padron, M.E.T.; Rodriguez, J.J.S. Methodologies for the extraction of phenolic compounds from environmental samples: New approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.K.; Thongklay, J.; Meunprasertdee, P.; Kornthattalim, P.; Kaaewmanee, T. A Comparison of Three Extraction Methods for Phenolic Compounds and Antioxidant Activities from Moringa oleifera Leaves. Chiang Mai J. Sci. 2018, 45, 2779–2789. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.B.; Ruslan, M.S.H. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Yen, N.T.H.; Quoc, L.P.T. Optimization of ultrasound-assisted extraction of phenolic compounds from fresh Moringa oleifera leaves with a response surface methodology and comparison with the Soxhlet extraction method. Bull. Chem. Soc. Ethiop. 2022, 36, 261–275. [Google Scholar] [CrossRef]
- Laskar, R.A.; Sk, I.; Roy, N.; Begum, N.A. Antioxidant activity of Indian propolis and its chemical constituents. Food Chem. 2010, 122, 233–237. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Petrus, J.C.C.; Hubinger, M.D. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J. Food Eng. 2010, 96, 533–539. [Google Scholar] [CrossRef]
- Jahan, S.; Shahjahan, M.; Rasna, S.S.; Aktar, M.; Sultana, S.; Ahmed, S.M.; Sabrin, F.; Nahar, S. Antibacterial Effect of Moringa (Moringa oleifera) Leaf Ethanolic Extract Against Staphylococcus aureus and Escherichia coli. Mymensingh Med. J. 2022, 31, 976–982. [Google Scholar]
- Magaji, U.F.; Sacan, O.; Yanardag, R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. S. Afr. J. Bot. 2020, 128, 225–230. [Google Scholar] [CrossRef]
- Niziol-Lukaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. Extracts as Bioactive Ingredients That Increase Safety of Body Wash Cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef]
- Gomes, S.M.; Leitão, A.; Alves, A.; Santos, L. Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules 2023, 28, 2526. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Adeoti, O.A.; Ariyo, O. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci. Nutr. 2013, 1, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices. Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Landázuri, A.C.; Gualle, A.; Castañeda, V.; Morales, E.; Caicedo, A.; Orejuela-Escobar, L.M. Moringa oleifera Lam. leaf powder antioxidant activity and cytotoxicity in human primary fibroblasts. Nat. Prod. Res. 2021, 35, 6194–6199. [Google Scholar] [CrossRef]
- Bukar, A.; Uba, A.; Oyeyi, T. Antimicrobial profile of Moringa oleifera lam. Extracts against some food—Borne microorganisms. Bayero J. Pure Appl. Sci. 2010, 3, 43–48. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Oyerinde, V.O.; Adeniyan, O.S. Physicochemical and Antioxidant Properties of Whole-Wheat Biscuits Incorporated with Moringa oleifera Leaves and Cocoa Powder. J. Sci. Res. Rep. 2015, 7, 195–206. [Google Scholar] [CrossRef]
- Jimenez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancin-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef]
- Abdel-Samie, M.A.S.; Abdulla, G. Effect of Moringa Leaves (Moringa oleifera Lam.) on Some Physico-Chemical and Sensory Properties of Wheat Flour Cookies. Zagazig J. Agric. Res. 2014, 41, 305–314. [Google Scholar]
- Srinivasamurthy, S.; Yadav, U.; Sahay, S.; Singh, A. Development of muffin by incorporation of dried Moringa oleifera (Drumstick) leaf powder with enhanced micronutrient content. Int. J. Food Sci. Nutr. 2017, 2, 173–178. [Google Scholar]
- Akinyeye, A.J. Phytochemical and antimicrobial evaluation of leaf and seed of Moringa oleifera extracts. Int. J. Res. Med. Health Sci. 2014, 4, 1–10. [Google Scholar]
- Aly, A.A.; Zaky, E.A.; Khatab, N.R.; Hameed, A.M.; Kadasah, S. The Biological and Chemical Ameliorative Effects of Bread Substituted with Dried Moringa Leaves. Arab. J. Chem. 2022, 15, 103875. [Google Scholar] [CrossRef]
- Govender, L.; Siwela, M. The Effect of Moringa oleifera Leaf Powder on the Physical Quality, Nutritional Composition and Consumer Acceptability of White and Brown Breads. Foods 2020, 9, 1910. [Google Scholar] [CrossRef]
- Ismawati, R.; Wahini, M.; Romadhoni, I.F.; Aina, Q. Sensory Preference, Nutrient Content, and Shelf Life of Moringa Oliefera Leaf Crackers. Int. J. Adv. Sci. Eng. Inf. 2019, 9, 489–494. [Google Scholar] [CrossRef]
- El-Gammal, R.; Ghoneim, G.; ElShehawy, S. Effect of Moringa Leaves Powder (Moringa oleifera) on Some Chemical and Physical Properties of Pan Bread. J. Food Dairy Sci. 2016, 7, 307–314. [Google Scholar] [CrossRef]
- Sengev, A.I.; Abu, J.O.; Gernah, D.I. Effect of Moringa oleifera Leaf Powder Supplementation on Some Quality Characteristics of Wheat Bread. Food Nutr. Sci. 2013, 4, 270–275. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) Flower as an Antioxidant Dietary Fibre in Chicken Meat Nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef]






| Product | Study Methods | Findings | Ref. |
|---|---|---|---|
| Dried pasta | Formulation of pasta containing semolina and 10%, 20%, 30%, and 40% of MOLP. The moisture, protein, and ash content of the samples were investigated. A sensory evaluation and a texture profile analysis were also conducted. | The pasta fortified with MOLP exhibited higher protein and ash contents in comparison to the control pasta and a lower moisture content. Regarding textural parameters and consumer acceptability, the pasta with 20% MOLP inclusion was found to be the best. | [22] |
| Bread | Assessment of the impact of MOPL addition at levels ranging from 0% to 10% on the proximate, mineral, antioxidant, and sensory attributes of WWF-leavened bread. | The MOLP-supplemented bread showed noticeable improvements in proximate and mineral profiles. The TPC of MOLP in comparison to WWF was much higher. Additionally, a 5% MOLP-based value-added bread demonstrated significantly higher antioxidant activities. The overall acceptability scores for WWF-leavened bread decreased progressively as MOLP addition levels increased. | [23] |
| Gluten-free bread | Fortification of rice semolina gluten-free bread with different amounts of MOLP (2.5%, 5%, 7.5%, and 10%). The TPC and antioxidant activity were determined. A texture and sensory analysis were also performed. | The addition of MOLP resulted in a significant decrease in the volume of the bread samples, except for the 2.5% MOLP. Additionally, a slight decrease in hardness and chewiness was observed with the addition of 2.5% and 10% MOLP. The TPC and antioxidant activity increased as the amount of MOLP increased. Among all MOLP-containing bread samples, the most acceptable bread was the one containing 2.5% MOLP in comparison to the control. | [24] |
| Biscuits | Production of biscuits with WWF substituted with MOLP (2.5%, 5%, and 10%). Evaluated the nutritional content, bioactive compounds, antioxidant, physical, and α-amylase inhibitory properties. The sensory attributes of the cookies were also determined. | MOLP-supplemented cookies had a significant enhancement in their bioactive compounds, antioxidant, and α-amylase inhibitory properties. Protein, ash, fat, and fibre contents were significantly increased in MOLP-substituted cookies. The sensory acceptance of the cookies decreased with increasing levels of WWF substitution. | [25] |
| Biscuits | Preparation of biscuits by substituting WWF with 5%, 10%, and 15% MOLP. The effect of MOLP on the rheological, microstructural, nutritional, textural, and organoleptic characteristics of biscuits was tested. | The addition of MOLP led to higher water absorption, softer dough, and an altered cookie texture. Sensory evaluation favoured cookies with 10% MOLP. Nutritional components like protein, iron, calcium, β-carotene, and dietary fibre increased with a higher MOLP content (0–15%). | [26] |
| Ingredient | NC-Br | MP-Br | ME-Br |
|---|---|---|---|
| Water (g) | 210 | 210 | 210 |
| Salt (g) | 4 | 4 | 4 |
| Wheat flour (g) | 360 | 342 | 352.55 |
| Yeast (g) | 2 | 2 | 2 |
| MOLP (g) | - | 18 | - |
| M. oleifera extract (g) | - | - | 7.45 |
| Ingredient | NC-Bi | MP-Bi | ME-Bi |
|---|---|---|---|
| Butter (g) | 125 | 125 | 125 |
| Sugar (g) | 125 | 125 | 125 |
| No. Eggs | 1 | 1 | 1 |
| Wheat flour (g) | 250 | 237.5 | 244.82 |
| Yeast (g) | 2 | 2 | 2 |
| MOLP (g) | - | 12.5 | - |
| M. oleifera extract (g) | - | - | 5.18 |
| Extraction Method | Extraction Conditions | Extraction Yield (%) | Ref. |
|---|---|---|---|
| UAE | Solvent: 70% ethanol w/v ratio: 1:40 Time: 30 min + 2.5 h Temperature: RT + 50 °C | 34.1 ± 0.9 | [28] |
| Sonication | Solvent: 80% ethanol w/v ratio: 1:50 Time: 30 min (×3) | 56.44 ± 0.82 | [34] |
| Maceration | Solvent: 50% or 70% ethanol w/v ratio: 1:40 Time: 72 h Temperature: RT | Et50: 38.34 ± 1.17 Et70: 40.50 ± 1.24 | [35] |
| Percolation | Solvent: 50% or 70% ethanol | Et50: 34.47 ± 1.41 Et70: 32.75 ± 1.93 | |
| Soxhlet | Solvent: 50% or 70% ethanol w/v ratio: 1:50 Time: 20 h | Et50: 33.58 ± 1.58 Et70: 35.87 ± 1.12 |
| TPC (mgGAE/gextract) | Antioxidant Capacity (IC50—mgextract/L) (TEAC—mgTE/gextract) | Antibacterial Capacity (dhalo—mm) | α-Amylase Inhibition Capacity (%) | ||
|---|---|---|---|---|---|
| 138.2 ± 17.0 | DPPH | ABTS | E. coli | S. aureus | 94.1 ± 0.4 |
| 544.0 ± 7.9 12.8 ± 0.2 | 115.2 ± 4.9 32.8 ± 1.4 | ND | ND | ||
| Compounds | RT (min) | Calibration Curves | R2 | IDL (mg/L) | IQL (mg/L) | Phenolic Concentration (mgcompound/gextract) |
|---|---|---|---|---|---|---|
| Catechin | 24.38 | A = 1.57 × 105 C − 7.93 × 105 | 0.9861 | 41.50 | 138.34 | 1.24 |
| Epicatechin | 30.34 | A = 4.15 × 105 C − 1.56 × 106 | 0.9983 | 5.82 | 19.39 | 0.09 |
| Caffeic acid | 29.23 | A = 5.56 × 105 C − 1.56 × 106 | 0.9992 | 4.03 | 13.43 | 0.07 |
| Chlorogenic acid | 26.62 | A = 1.91 × 105 C − 1.16 × 105 | 0.9999 | 2.84 | 9.48 | 0.01 |
| Quercetin | 52.79 | A = 7.37 × 105 C − 2.68 × 105 | 0.9994 | 1.37 | 4.58 | 0.01 |
| Formulation | Timepoint | LSA | RBC |
|---|---|---|---|
| (CFU/mL) | |||
| NC-Br | t0 | ND | ND |
| t1 | >30 × 105 | >30 × 105 | |
| MP-Br | t0 | ND | ND |
| t1 | >30 × 105 | ND | |
| ME-Br | t0 | ND | ND |
| t1 | 3.05 × 105 | ND | |
| NC-Bi | t0 | ND | ND |
| t1 | ND | ND | |
| t2 | ND | ND | |
| t3 | ND | ND | |
| MP-Bi | t0 | ND | ND |
| t1 | ND | ND | |
| t2 | ND | ND | |
| t3 | ND | ND | |
| ME-Bi | t0 | ND | ND |
| t1 | ND | ND | |
| t2 | ND | ND | |
| t3 | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.; Gomes, S.M.; Santos, L. Elevating Cereal-Based Nutrition: Moringa oleifera Supplemented Bread and Biscuits. Antioxidants 2023, 12, 2069. https://doi.org/10.3390/antiox12122069
Ferreira T, Gomes SM, Santos L. Elevating Cereal-Based Nutrition: Moringa oleifera Supplemented Bread and Biscuits. Antioxidants. 2023; 12(12):2069. https://doi.org/10.3390/antiox12122069
Chicago/Turabian StyleFerreira, Teresa, Sandra M. Gomes, and Lúcia Santos. 2023. "Elevating Cereal-Based Nutrition: Moringa oleifera Supplemented Bread and Biscuits" Antioxidants 12, no. 12: 2069. https://doi.org/10.3390/antiox12122069