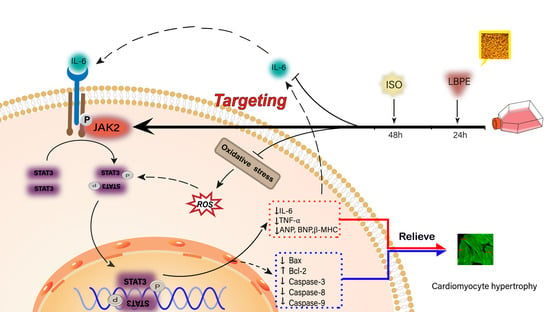
Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. LBPE Preparation and Ultra-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QTOF MS) Analysis
2.3. Cell Culture and Group Administration
2.4. Morphological Analysis
2.5. Determination of Cellular Antioxidant Capacity and Total Protein Content
2.6. Quantitative Real-Time PCR
2.7. Target Fishing, Bioinformatic Analysis and Molecular Docking
2.8. Blocking p-JAK2 by AG490
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Identification of the Main Components in LBPE
3.2. Effects of LBPE on the Morphology, Surface Area and Total Protein Content of H9c2 Cells Induced by ISO
3.3. Effect of LBPE on ANP, BNP and β-MHC mRNA Expression Levels in rat H9c2 Cells Injured by ISO
3.4. Effects of LBPE on MDA, SOD and GSH Levels in H9c2 Cells Injured by ISO
3.5. Effects of LBPE on Inflammation in H9c2 Cells Injured by ISO
3.6. Effects of LBPE on Apoptosis in H9c2 Cells Injured by ISO
3.7. Targets and Effect Pathways Prediction for LBPE against CH
3.8. Effect of LBPE on JAK2/STAT3 Signal Pathway in H9c2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Hu, C.; Zhao, X.; Zhang, P.; Chang, Y.; Shang, Y.; Pang, Y.; Qian, W.; Qiu, X.; et al. Lingguizhugan decoction dynamically regulates MAPKs and AKT signaling pathways to retrogress the pathological progression of cardiac hypertrophy to heart failure. Phytomedicine 2022, 98, 153951. [Google Scholar] [CrossRef] [PubMed]
- Lopes Fernandes, S.; Ribeiro Carvalho, R.; Graça Santos, L.; Montenegro Sá, F.; Ruivo, C.; Lázaro Mendes, S.; Martins, H.; Araujo Morais, J. Pathophysiology and treatment of heart failure with preserved ejection fraction: State of the art and prospects for the future. Arq. Bras. Cardiol. 2019, 141, 120–129. [Google Scholar]
- Chen, L.F.; Meng, F.; Li, Z.L.; Xie, S.H.; Shi, P.Y. Study on the protective effect and mechanism of rape bee pollens ethanol extract on cardiomyocyte hypertrophy induced by isoproterenol. Nat. Prod. Res. Dev. 2021, 33, 1741–1750. [Google Scholar]
- Chowdhury, D.; Kumar, D.; Bhadra, U.; Devi, T.A.; Bhadra, M.P. Prohibitin confers cytoprotection against ISO-induced hypertrophy in H9c2 cells via attenuation of oxidative stress and modulation of Akt/Gsk-3β signaling. Mol. Cell. Biochem. 2017, 425, 155–168. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Shi, P.; Geng, Q.; Chen, L.; Du, T.; Lin, Y.; Lai, R.; Meng, F.; Wu, Z.; Miao, X.; Yao, H. Schisandra chinensis bee pollen’s chemical profiles and protective effect against H2O2-induced apoptosis in H9c2 cardiomyocytes. BMC Complement. Med. Ther. 2020, 20, 274. [Google Scholar] [CrossRef]
- Pimentel, J.D.R.; Oliveira, T.V.; Souza, D.S.; Nunes, M.L.; Narain, N.; Da Silva, M.A.A.P. Post-harvest conservation of mangaba fruit by application of biofilm containing cassava starch and chitosan. Acta Hortic. 2018, 1198, 251–254. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Lu, J.; Hong, H.; Yuan, J.; Zhang, Y.; Wang, P.; Liu, P.; Ye, J. Protocatechuic aldehyde protects against isoproterenol-induced cardiac hypertrophy via inhibition of the JAK2/STAT3 signaling pathway. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 1373–1385. [Google Scholar] [CrossRef]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK-STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.W.; Sun, J.H.; Yang, H.X.; Xu, G.R.; Zhang, Y.; Song, Q.H.; Zhang, C.; Liu, W.Z.; Liu, X.C.; et al. Modified citrus pectin prevents isoproterenol-induced cardiac hypertrophy associated with p38 signalling and TLR4/JAK/STAT3 pathway. Biomed. Pharmacother. 2021, 143, 112178. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, X.C.; Wang, L.; Cui, D.A.; Zhang, J.Y.; Wang, X.R.; Feng, H.P.; Zhang, K.; Zhang, K.; Li, J.X.; et al. Schisandrin protects against norepinephrine-induced myocardial hypertrophic injury by inhibiting the JAK2/STAT3 signaling pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 129512. [Google Scholar] [CrossRef] [PubMed]
- Zouein, F.A.; Kurdi, M.; Booz, G.W. Dancing rhinos in stilettos: The amazing saga of the genomic and nongenomic actions of STAT3 in the heart. Jak-Stat 2013, 2, e24352. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Li, P.; Zheng, M.; Chen, L.; Xu, Q.; Chen, K.; Wang, Y.Y.; Zhang, Y.Y.; Han, C. Interleukin-6 family of cytokines mediates isoproterenol-induced delayed STAT3 activation in mouse heart. J. Biol. Chem. 2003, 278, 21070–21075. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Liu, Z.; Lin, Z.; Shi, P.; Chen, B.; Li, S.; Li, G.; Huang, L.; Lin, X.; Yao, H. Potential mechanism of action of Ixeris sonchifolia extract injection against cardiovascular diseases revealed by combination of HPLC-Q-TOF-MS, virtual screening and systems pharmacology approach. RSC Adv. 2020, 10, 38497–38504. [Google Scholar] [CrossRef]
- Xie, R.F.; Lin, Z.; Zhong, C.H.; Li, S.G.; Chen, B.; Wu, Y.J.; Huang, L.Y.; Yao, H.; Shi, P.Y.; Huang, J.Y. Deciphering the potential anti-COVID-19 active ingredients in Andrographis paniculata (Burm. F.) Nees by combination of network pharmacology, molecular docking, and molecular dynamics. RSC Adv. 2021, 11, 36511–36517. [Google Scholar] [CrossRef]
- Yao, H.; Huang, X.M.; Xie, Y.J.; Huang, X.L.; Ruan, Y.J.; Lin, X.H.; Huang, L.Y.; Shi, P.Y. Identification of pharmacokinetic markers for Guanxin Danshen drop pills in rats by combination of pharmacokinetics, systems pharmacology, and pharmacodynamic assays. Front. Pharmacol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Kwapisz, A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. Nat. Prod. Res. 2011, 25, 1115–1131. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.X.; Zhang, Y.J.; Yang, Y.H.; Lu, M.L.; Zhang, J.; Li, S.T.; Zhang, S.P.; Li, G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-κB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef]
- Puhl, S.L.; Kazakov, A.; Müller, A.; Fries, P.; Wagner, D.R.; Böhm, M.; Maack, C.; Devaux, Y. Adenosine A1 receptor activation attenuates cardiac hypertrophy and fibrosis in response to α1-adrenoceptor stimulation in vivo. Br. J. Pharmacol. 2016, 173, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, K.; Nishimura, A.; Sunggip, C.; Ito, T.; Nishiyama, K.; Kato, Y.; Tanaka, T.; Tozaki-Saitoh, H.; Tsuda, M.; Nishida, M. Modulation of P2Y6R expression exacerbates pressure overload-induced cardiac remodeling in mice. Sci. Rep. 2020, 10, 13926. [Google Scholar] [CrossRef] [PubMed]
- Adzika, G.K.; Hou, H.; Adekunle, A.O.; Rizvi, R.; Adu-Amankwaah, J.; Shang, W.; Li, K.; Deng, Q.M.; Mprah, R.; Noah, M.L.N.; et al. Isoproterenol-induced cardiomyopathy recovery intervention: Amlexanox and forskolin enhances the resolution of catecholamine stress-induced maladaptive myocardial remodeling. Front. Cardiovasc. Med. 2021, 8, 719805. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Z.; Chen, D.L. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed. Pharmacother. 2017, 93, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, D.; Sang, M.; Xu, Y.; Liu, Z.; Wu, Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-κB and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2017, 48, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Jagadeesh, G. Multifarious molecular signaling cascades of cardiac hypertrophy: Can the muddy waters be cleared? Pharmacol. Res. 2010, 62, 365–383. [Google Scholar] [CrossRef]
- Wincewicz, A.; Sulkowski, S. Stat proteins as intracellular regulators of resistance to myocardial injury in the context of cardiac remodeling and targeting for therapy. Adv. Clin. Exp. Med. 2017, 26, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Qu, X.; Chen, B.; Snyder, M.; Wang, M.; Li, B.; Tang, Y.; Chen, H.; Zhu, W.; Zhan, L.; et al. Critical roles of STAT3 in β-adrenergic functions in the heart. Circulation 2016, 133, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol attenuates angiotensin II-induced cardiac remodeling by targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Y.; Wang, Y.; Yang, T.; Feng, Z.; Qu, Y.; Zhou, H. Luhong formula inhibits myocardial fibrosis in a paracrine manner by activating the gp130/JAK2/STAT3 pathway in cardiomyocytes. J. Ethnopharmacol. 2017, 202, 28–37. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Duhamel, T.A.; Dhalla, N.S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can. J. Physiol. Pharmacol. 2020, 98, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, H.; Neukirchen, W.; Rahimi, G.; Grünheck, F.; Hescheler, J.; Wartenberg, M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp. Cell Res. 2004, 294, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Zhou, Y.; Liang, C.L.; Zhang, X.J.; Lai, J.M.; Ye, S.F.; Ouyang, H.; Lin, J.; Zhou, J.Y. Cyclovirobuxinum D alleviates cardiac hypertrophy in hyperthyroid rats by preventing apoptosis of cardiac cells and inhibiting the p38 mitogen-activated protein kinase signaling pathway. Chin. J. Integr. Med. 2017, 23, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, B.; Wu, C.; Wu, H.; Wu, J.; Wu, S.; Zhang, J.; Yang, X.; Yang, L.; Hu, Z.; et al. Plantago asiatica L. seeds extract protects against cardiomyocyte injury in isoproterenol- induced cardiac hypertrophy by inhibiting excessive autophagy and apoptosis in mice. Phytomedicine 2021, 91, 153681. [Google Scholar] [CrossRef] [PubMed]








| Name | Accession No. | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) |
|---|---|---|---|
| GAPDH | NM_017008 | AGCCCAGAACATCATCCCTG | ACGGATACATTGGGGGTAGG |
| ANP | NM_012612 | GGGAAGTCAACCCGTCTCAG | CAATCCTACCCCCGAAGCAG |
| BNP | NM_031545 | AGCTCTCAAAGGACCAAGGC | TCCGGTCTATCTTCTGCCCA |
| β-MHC | NM_017240 | ATCAAGGGAAAGCAGGAAGC | CCTTGTCTACAGGTGCATCA |
| Bax | NM_017059 | AGGATCGAGCAGAGAGGATG | AGCTCCATGTTGTTGTCCAGT |
| Bcl-2 | NM_016993 | GGGGCTACGAGTGGGATACT | GACGGTAGCGACGAGAGAAG |
| Caspase-3 | NM_012922 | CGGACCTGTGGACCTGAAAA | TAACCGGGTGCGGTAGAGTA |
| Caspase-8 | NM_022277 | CATCCTGACTGGCGTGAACT | TGGCATCTGCTTTCCCATGT |
| Caspase-9 | NM_031632 | GAGGATATTCAGCGGGCAGG | GCAGGAGATGAAGCGAGGAA |
| IL-6 | NM_012589 | TTCCAGCCAGTTGCCTTCTT | CTGGTCTGTTGTGGGTGGTA |
| TNF-α | NM_012675 | TCGTAGCAAACCACCAAGCA | GGTGAGGAGCACATAGTCGG |
| Group | SOD (U∙mL−1) | GSH (µmol∙g prot−1) | MDA (nmol∙mg prot−1) |
|---|---|---|---|
| Negative control | 25.6207 ± 2.9961 ## | 40.1040 ± 5.4200 ## | 0.9942 ± 0.1906 ## |
| ISO | 15.8183 ± 1.1401 | 14.9001 ± 3.6260 | 1.6708 ± 0.0870 |
| Positive | 23.9008 ± 0.7505 ## | 44.1822 ± 1.0518 ## | 1.0640 ± 0.1397 ## |
| 100 µg∙mL−1 LBPE | 17.2687 ± 3.1454 | 17.4918 ± 6.4598 | 1.3534 ± 0.0919 # |
| 250 µg∙mL−1 LBPE | 19.9866 ± 0.9251 | 27.1641 ± 11.6397 | 1.1742 ± 0.1502 ## |
| 500 µg∙mL−1 LBPE | 22.3155 ± 4.3936 ## | 32.3704 ± 6.1971 # | 1.1179 ± 0.1311 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Chen, L.; Zhang, Y.; Xie, S.; Yang, J.; Su, S.; Yao, H.; Shi, P. Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells. Antioxidants 2023, 12, 88. https://doi.org/10.3390/antiox12010088
Han S, Chen L, Zhang Y, Xie S, Yang J, Su S, Yao H, Shi P. Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells. Antioxidants. 2023; 12(1):88. https://doi.org/10.3390/antiox12010088
Chicago/Turabian StyleHan, Shuo, Lifu Chen, Yi Zhang, Shihui Xie, Jiali Yang, Songkun Su, Hong Yao, and Peiying Shi. 2023. "Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells" Antioxidants 12, no. 1: 88. https://doi.org/10.3390/antiox12010088