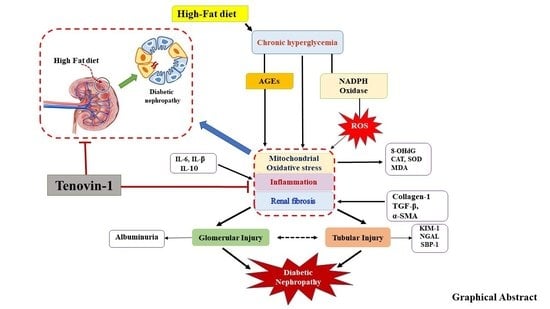
Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Materials
2.2. Experimental Design
2.3. Analysis of Serum Biochemical Parameters
2.4. Analysis of Urinary Parameters
2.5. Assessment of Oxidative Stress
2.6. Assessment of Diabetic Renal Inflammation
2.7. Western Blot Analysis
2.8. Determination of 4-Hydroxyproline Concentration
2.9. Analysis of the Concentration of 3-IS
2.10. Histopathological Examinations
2.11. Immunohistochemistry and Immunofluorescence Analysis
2.12. TUNEL Assay
2.13. mRNA Expression Analyses
2.14. Cell Line and Cell Culture
2.15. DAPI Nuclear Staining
2.16. Annexin V-FITC Binding Assay
2.17. Cell Cycle Analysis
2.18. Statistical Analysis
3. Results
3.1. Effect of Tenovin-1 on Blood Glucose and Body Weight
3.2. Effect of Tenovin-1 on the Weight of Major Organs and Histological Results in ZDF Rats Fed an HFD
3.3. Effect of Tenovin-1 on AGEs and Serum Biochemical Parameters
3.4. Effect of Tenovin-1 on Urinary Biochemical Parameters
3.5. Effect of Tenovin-1 on Oxidative Biomarkers of Diabetic Rats
3.6. Effect of Tenovin-1 on Inflammatory Cytokines of Diabetic Rats
3.7. Effect of Tenovin-1 on Urinary Biomarkers in HFD-Induced ZDF Rats
3.8. Effect of Tenovin-1 on Renal Fibrosis in HFD-Induced ZDF Rats and NRK-52E Cell Line
3.9. Effects of Tenovin-1 on Renal Apoptosis in Diabetic Rats and HG-Induced NRK-52E Cell Line
3.10. Effect of Tenovin-1 on Sirtuins and Claudin-1 in Renal Cortexes of HFD-Fed Rats
3.11. Effect of Tenovin-1 on the Phosphorylation Levels of EGFR, PDGFRβ, and STAT3 in Kidneys of HFD-Induced ZDF Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The Worldwide Epidemiology of Type 2 Diabetes Mellitus—Present and Future Perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Zhang, Z.; Mima, A.; King, G.L. Molecular Mechanisms of Diabetic Vascular Complications. J. Diabetes Investig. 2010, 1, 77–89. [Google Scholar] [CrossRef]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats by Punica Granatum Linn. Leaves Extract. J. Tradit. Complement. Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Badid, C.; Desmouliere, A.; Babici, D.; Hadj-Aissa, A.; McGregor, B.; Lefrancois, N.; Touraine, J.L.; Laville, M. Interstitial Expression of α-SMA: An Early Marker of Chronic Renal Allograft Dysfunction. Nephrol. Dial. Transplant. 2002, 17, 1993–1998. [Google Scholar] [CrossRef]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic Nephropathy and Extracellular Matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kim, K.S.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L.; et al. Protective Effects of Croton Hookeri on Streptozotocin-Induced Diabetic Nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef]
- Mason, R.M.; Wahab, N.A. Extracellular Matrix Metabolism in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef]
- Peck, B.; Chen, C.Y.; Ho, K.K.; Di Fruscia, P.; Myatt, S.S.; Charles Coombes, R.; Fuchter, M.J.; Hsiao, C.D.; Lam, E.W.F. SIRT Inhibitors Induce Cell Death and P53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Lain, S.; Hollick, J.J.; Campbell, J.; Staples, O.D.; Higgins, M.; Aoubala, M.; McCarthy, A.; Appleyard, V.; Murray, K.E.; Baker, L.; et al. Discovery, In Vivo Activity, and Mechanism of Action of a Small-Molecule P53 Activator. Cancer Cell 2008, 13, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.J.; Singh, C.; Nihal, M.; Zhong, W.; Ahmad, N. SIRT1 Deacetylase Is Overexpressed in Human Melanoma and Its Small Molecule Inhibition Imparts Anti-Proliferative Response via P53 Activation. Arch. Biochem. Biophys. 2014, 563, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.J.; Singh, C.K.; Nihal, M.; Ndiaye, M.A.; Ahmad, N. Sirtuin Deacetylases: A New Target for Melanoma Management. Cell Cycle 2014, 13, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Dey, P.; Park, J.H.; Kim, I.S.; Kwack, S.J.; Kim, H.S. EX-527 Prevents the Progression of High-Fat Diet-Induced Hepatic Steatosis and Fibrosis by Upregulating SIRT4 in Zucker Rats. Cells 2020, 9, 1101. [Google Scholar] [CrossRef]
- Ponnusamy, M.; Zhou, X.; Yan, Y.; Tang, J.; Tolbert, E.; Zhao, T.C.; Gong, R.; Zhuang, S. Blocking Sirtuin 1 and 2 Inhibits Renal Interstitial Fibroblast Activation and Attenuates Renal Interstitial Fibrosis in Obstructive Nephropathy. J. Pharmacol. Exp. Ther. 2014, 350, 243–256. [Google Scholar] [CrossRef]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.Y.; Lee, B.H.; Lee, K.Y.; Kacew, S.; et al. Protective Effect of EX-527 against High-Fat Diet-Induced Diabetic Nephropathy in Zucker Rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gassler, N.; Elger, M.; Kränzlin, B.; Kriz, W.; Gretz, N.; Hähnel, B.; Hosser, H.; Hartmann, I. Podocyte Injury Underlies the Progression of Focal Segmental Glomerulosclerosis in the Fa/Fa Zucker Rat. Kidney Int. 2001, 60, 106–116. [Google Scholar] [CrossRef]
- Mora, C.; Navarro, J.F. Inflammation and Diabetic Nephropathy. Curr. Diab. Rep. 2006, 6, 463–468. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic Effect of Ginkgo Biloba Extract in Streptozotocin-Induced Diabetes in Rats. Biomed. Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal Tubular Sirt1 Attenuates Diabetic Albuminuria by Epigenetically Suppressing Claudin-1 Overexpression in Podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, O.; Jasiek, M.; Hénique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial Cell and Podocyte Autophagy Synergistically Protect from Diabetes-Induced Glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef]
- Saravanan, S.; Pari, L. Protective Effect of Thymol on High Fat Diet Induced Diabetic Nephropathy in C57BL/6J Mice. Chem. Biol. Interact. 2016, 245, 1–11. [Google Scholar] [CrossRef]
- Wang, G.G.; Lu, X.H.; Li, W.; Zhao, X.; Zhang, C. Protective Effects of Luteolin on Diabetic Nephropathy in STZ-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2011, 2011, 323171. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.; Pippin, J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Etgen, G.J.; Oldham, B.A. Profiling of Zucker Diabetic Fatty Rats in Their Progression to the Overt Diabetic State. Metabolism 2000, 49, 684–688. [Google Scholar] [CrossRef]
- Siwy, J.; Zoja, C.; Klein, J.; Benigni, A.; Mullen, W.; Mayer, B.; Mischak, H.; Jankowski, J.; Stevens, R.; Vlahou, A.; et al. Evaluation of the Zucker Diabetic Fatty (ZDF) Rat as a Model for Human Disease Based on Urinary Peptidomic Profiles. PLoS ONE 2012, 7, e51334. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A. Zucker Diabetic Fatty Rats for Research in Diabetes. Anim. Model. Med. Biol. 2020, 1–18. [Google Scholar] [CrossRef]
- Magil, A.B. Tubulointerstitial Lesions in Young Zucker Rats. Am. J. Kidney Dis. 1995, 25, 478–485. [Google Scholar] [CrossRef]
- Coimbra, T.M.; Janssen, U.; Gröne, H.J.; Ostendorf, T.; Kunter, U.; Schmidt, H.; Brabant, G.; Floege, J. Early Events Leading to Renal Injury in Obese Zucker (Fatty) Rats with Type II Diabetes. Kidney Int. 2000, 57, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, S.; Shu, Y.; Yoshida, F.; Inagaki, T.; Sonoda, J.; Watanabe, T.; Nomoto, K.I.; Nagata, M. Podocyte Injury Promotes Progressive Nephropathy in Zucker Diabetic Fatty Rats. Lab. Investig. 2002, 82, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.G.; Shaw, W.N.; Neel, M.-A.; Little, L.A.; Eichberg, J. Zucker Diabetic Fatty Rat as a Model for Non-Insulin-Dependent Diabetes Mellitus. ILAR J. 1990, 32, 16–19. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Monno, I.; Kanasaki, K.; Watanabe, A.; Koya, D. Renal Mitochondrial Oxidative Stress Is Enhanced by the Reduction of Sirt3 Activity, in Zucker Diabetic Fatty Rats. Redox Rep. 2018, 23, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Hoshino, M.; Hidaka, S.; Yoshida, E.; Beppu, M.; Hoshikawa, R.; Sudo, K.; Kawada, A.; Takagi, S.; Seino, S. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J. Diabetes Res. 2013, 2013, 103731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Chen, C.; Dumlao, T.; Naik, S.; Chang, T.; Xiao, Y.Y.; Sominsky, I.; Burton, J. Enhanced Histone Deacetylase Enzyme Activity in Primary Myelofibrosis. Leuk. Lymphoma 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Ponnusamy, M.; Zhuang, M.A.; Zhou, X.; Tolbert, E.; Bayliss, G.; Zhao, T.C.; Zhuang, S. Activation of Sirtuin-1 Promotes Renal Fibroblast Activation and Aggravates Renal Fibrogenesis. J. Pharmacol. Exp. Ther. 2015, 354, 142–151. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, K.S.; Park, J.H.; Lee, S.H.; Kim, H.R.; Lee, S.H.; Choi, H.B.; Cao, S.; Kumar, V.; Kwak, J.H.; et al. Protective Effects of Dendropanoxide Isolated from Dendropanax Morbifera against Cisplatin-Induced Acute Kidney Injury via the AMPK/MTOR Signaling Pathway. Food Chem. Toxicol. 2020, 145, 111605. [Google Scholar] [CrossRef] [PubMed]
- He, F.F.; You, R.Y.; Ye, C.; Lei, C.T.; Tang, H.; Su, H.; Zhang, C. Inhibition of SIRT2 Alleviates Fibroblast Activation and Renal Tubulointerstitial Fibrosis via MDM2. Cell. Physiol. Biochem. 2018, 46, 451–460. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, A.S.; Nguyen-Thanh, T.; Kim, D.; Kang, K.P.; Lee, S.; Park, S.K.; Kim, W. SIRT2 Regulates LPS-Induced Renal Tubular CXCL2 and CCL2 Expression. J. Am. Soc. Nephrol. 2015, 26, 1549–1560. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Zhang, M.Z.; You, L.; Davis, L.S.; Fan, H.; Yang, H.C.; Fogo, A.B.; Zent, R.; Harris, R.C.; et al. Sirt1 Activation Protects the Mouse Renal Medulla from Oxidative Injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef] [Green Version]
- Villalba, J.M.; Alcaín, F.J. Sirtuin Activators and Inhibitors. BioFactors 2012, 38, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yang, H.C.; You, L.; Wang, Y.Y.; He, W.J.; Hao, C.M. The Histone Deacetylase, SIRT1, Contributes to the Resistance of Young Mice to Ischemia/Reperfusion-Induced Acute Kidney Injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.C.; Atangan, L.; Wang, M. Resveratrol Is Not a Direct Activator of Sirt1 Enzyme Activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R. An UnSIRTain Role in Longevity. Nat. Med. 2011, 17, 1350–1351. [Google Scholar] [CrossRef]
- Liu, R.; Zhong, Y.; Li, X.; Chen, H.; Jim, B.; Zhou, M.M.; Chuang, P.Y.; He, J.C. Role of Transcription Factor Acetylation in Diabetic Kidney Disease. Diabetes 2014, 63, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Monday, O.M.; Uzoma, A.I. Histological Changes and Antidiabetic Activities of Icacina Trichantha Tuber Extract in Beta-Cells of Alloxan Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2013, 3, 628–633. [Google Scholar] [CrossRef]
- Junod, A.; Lambert, A.E.; Stauffacher, W.; Renold, A.E. Diabetogenic Action of Streptozotocin: Relationship of Dose to Metabolic Response. J. Clin. Investig. 1969, 48, 2129–2139. [Google Scholar] [CrossRef]
- Soler, M.J.; Riera, M.; Batlle, D. New Experimental Models of Diabetic Nephropathy in Mice Models of Type 2 Diabetes: Efforts to Replicate Human Nephropathy. Exp. Diabetes Res. 2012, 2012, 616313. [Google Scholar] [CrossRef]
- Hsu, J.D.; Wu, C.C.; Hung, C.N.; Wang, C.J.; Huang, H.P. Myrciaria Cauliflora Extract Improves Diabetic Nephropathy via Suppression of Oxidative Stress and Inflammation in Streptozotocin-Nicotinamide Mice. J. Food Drug Anal. 2016, 24, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, V.; Snehalatha, C.; Kumutha, R.; Jayaraman, M.; Ramachandran, A. Serum Albumin Levels in Different Stages of Type 2 Diabetic Nephropathy Patients. Indian J. Nephrol. 2004, 14, 89–92. [Google Scholar]
- Xue, W.; Lei, J.; Li, X.; Zhang, R. Trigonella Foenum Graecum Seed Extract Protects Kidney Function and Morphology in Diabetic Rats via Its Antioxidant Activity. Nutr. Res. 2011, 31, 555–562. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of Acute Kidney Injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Fiseha, T. Urinary Biomarkers for Early Diabetic Nephropathy in Type 2 Diabetic Patients. Biomark. Res. 2015, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Yang, H.Y.; Song, H.; Kang, Y.R.; Kwon, J.H.; An, J.H.; Son, J.Y.; Kwack, S.J.; Kim, Y.M.; Bae, O.N.; et al. Identification of a Sensitive Urinary Biomarker, Selenium-Binding Protein 1, for Early Detection of Acute Kidney Injury. J. Toxicol. Environ. Health Part A Curr. Issues 2017, 80, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Young, G.H.; Huang, P.H.; Lo, S.C.; Wang, K.C.; Sun, C.Y.; Liang, C.J.; Huang, T.M.; Chen, J.H.; Chang, F.C.; et al. In Acute Kidney Injury, Indoxyl Sulfate Impairs Human Endothelial Progenitor Cells: Modulation by Statin. Angiogenesis 2013, 16, 609–624. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Sagata, M.; Saigo, C.; Yoneda, G.; Yamamoto, Y.; Nomura, Y.; Nishi, K.; Fujino, R.; Jono, H.; Saito, H. Indoxyl Sulfate as a Mediator Involved in Dysregulation of Pulmonary Aquaporin-5 in Acute Lung Injury Caused by Acute Kidney Injury. Int. J. Mol. Sci. 2017, 18, 11. [Google Scholar] [CrossRef]
- Won, A.J.; Kim, S.; Kim, Y.G.; Kim, K.B.; Choi, W.S.; Kacew, S.; Kim, K.S.; Jung, J.H.; Lee, B.M.; Kim, S.; et al. Discovery of Urinary Metabolomic Biomarkers for Early Detection of Acute Kidney Injury. Mol. Biosyst. 2016, 12, 133–144. [Google Scholar] [CrossRef]
- King, G.L.; Loeken, M.R. Hyperglycemia-Induced Oxidative Stress in Diabetic Complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef]
- Gujjala, S.; Putakala, M.; Nukala, S.; Bangeppagari, M.; Ramaswamy, R.; Desireddy, S. Renoprotective Effect of Caralluma Fimbriata against High-Fat Diet-Induced Oxidative Stress in Wistar Rats. J. Food Drug Anal. 2016, 24, 586–593. [Google Scholar] [CrossRef]
- Niedowicz, D.M.; Daleke, D.L. The Role of Oxidative Stress in Diabetic Complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Gimeno, C.J.; Ames, B.N. Urinary 8-Hydroxy-2′-Deoxyguanosine as a Biological Marker of in Vivo Oxidative DNA Damage. Proc. Natl. Acad. Sci. USA 1989, 86, 9697–9701. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative Stress, Nutrition and Health. Experimental Strategies for Optimization of Nutritional Antioxidant Intake in Humans. Free Radic. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Hinokio, Y.; Suzuki, S.; Hirai, M.; Suzuki, C.; Suzuki, M.; Toyota, T. Urinary Excretion of 8-Oxo-7, 8-Dihydro-2′-Deoxyguanosine as a Predictor of the Development of Diabetic Nephropathy. Diabetologia 2002, 45, 877–882. [Google Scholar] [CrossRef]
- Xu, G.W.; Yao, Q.H.; Weng, Q.F.; Su, B.L.; Zhang, X.; Xiong, J.H. Study of Urinary 8-Hydroxydeoxyguanosine as a Biomarker of Oxidative DNA Damage in Diabetic Nephropathy Patients. J. Pharm. Biomed. Anal. 2004, 36, 101–104. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Q.; Guo, F.; Zhao, Y.; Ji, L.; An, T.; Song, Y.; Liu, Y.; He, Y.; Qin, G. FoxO1-Mediated Inhibition of STAT1 Alleviates Tubulointerstitial Fibrosis and Tubule Apoptosis in Diabetic Kidney Disease. EBioMedicine 2019, 48, 491–504. [Google Scholar] [CrossRef]
- Portilla, D. Apoptosis, Fibrosis and Senescence. Nephron Clin. Pract. 2014, 127, 65–69. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, F.; Li, Y.; Gao, L.; Zhang, J. The Protective Effect of Glycyrrhizic Acid on Renal Tubular Epithelial Cell Injury Induced by High Glucose. Int. J. Mol. Sci. 2014, 15, 15026–15043. [Google Scholar] [CrossRef]
- Zhao, T.T.; Zhang, H.J.; Lu, X.G.; Huang, X.R.; Zhang, W.K.; Wang, H.; Lan, H.Y.; Li, P. Chaihuang-Yishen Granule Inhibits Diabetic Kidney Disease in Rats through Blocking TGF-β/Smad3 Signaling. PLoS ONE 2014, 9, e90807. [Google Scholar] [CrossRef]
- Böttinger, E.P.; Bitzer, M. TGF-β Signaling in Renal Disease. J. Am. Soc. Nephrol. 2002, 13, 2600–2610. [Google Scholar] [CrossRef]
- Liu, Y. Renal Fibrosis: New Insights into the Pathogenesis and Therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-Inflammasome Activation in Non-Myeloid-Derived Cells Aggravates Diabetic Nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef]
- Mu, W.; Ouyang, X.; Agarwal, A.; Zhang, L.; Long, D.A.; Cruz, P.E.; Roncal, C.A.; Glushakova, O.Y.; Chiodo, V.A.; Atkinson, M.A.; et al. IL-10 Suppresses Chemokines, Inflammation, and Fibrosis in a Model of Chronic Renal Disease. J. Am. Soc. Nephrol. 2005, 16, 3651–3660. [Google Scholar] [CrossRef]
- Lu, Q.; Zuo, W.Z.; Ji, X.J.; Zhou, Y.X.; Liu, Y.Q.; Yao, X.Q.; Zhou, X.Y.; Liu, Y.W.; Zhang, F.; Yin, X.X. Ethanolic Ginkgo Biloba Leaf Extract Prevents Renal Fibrosis through Akt/MTOR Signaling in Diabetic Nephropathy. Phytomedicine 2015, 22, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and Molecular Mechanisms in Fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Terzi, F.; Burtin, M.; Hekmati, M.; Federici, P.; Grimber, G.; Briand, P.; Friedlander, G. Targeted Expression of a Dominant-Negative EGF-R in the Kidney Reduces Tubulo-Interstitial Lesions after Renal Injury. J. Clin. Investig. 2000, 106, 225–234. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and Its Receptors in Fibrotic Diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Lautrette, A.; Li, S.; Alili, R.; Sunnarborg, S.W.; Burtin, M.; Lee, D.C.; Friedlander, G.; Terzi, F. Angiotensin II and EGF Receptor Cross-Talk in Chronic Kidney Diseases: A New Therapeutic Approach. Nat. Med. 2005, 11, 867–874. [Google Scholar] [CrossRef]
- Zhuang, S.; Dang, Y.; Schnellmann, R.G. Requirement of the Epidermal Growth Factor Receptor in Renal Epithelial Cell Proliferation and Migration. Am. J. Physiol. Ren. Physiol. 2004, 287, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chen, J.K.; Harris, R.C. Deletion of the Epidermal Growth Factor Receptor in Renal Proximal Tubule Epithelial Cells Delays Recovery from Acute Kidney Injury. Kidney Int. 2012, 82, 45–52. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, N.; Bayliss, G.; Zhuang, S. EGFR Activity Is Required for Renal Tubular Cell Dedifferentiation and Proliferation in a Murine Model of Folic Acid-Induced Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2013, 304, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.K.; Wang, S.W.; Moeckel, G.; Harris, R.C. Importance of Functional EGF Receptors in Recovery from Acute Nephrotoxic Injury. J. Am. Soc. Nephrol. 2003, 14, 3147–3154. [Google Scholar] [CrossRef]
- Pang, M.; Kothapally, J.; Mao, H.; Tolbert, E.; Ponnusamy, M.; Chin, Y.E.; Zhuang, S. Inhibition of Histone Deacetylase Activity Attenuates Renal Fibroblast Activation and Interstitial Fibrosis in Obstructive Nephropathy. Am. J. Physiol. Ren. Physiol. 2009, 297, 996–1005. [Google Scholar] [CrossRef]
- Kuratsune, M.; Masaki, T.; Hirai, T.; Kiribayashi, K.; Yokoyama, Y.; Arakawa, T.; Yorioka, N.; Kohno, N. Signal Transducer and Activator of Transcription 3 Involvement in the Development of Renal Interstitial Fibrosis after Unilateral Ureteral Obstruction. Nephrology 2007, 12, 565–571. [Google Scholar] [CrossRef]
- Zhuang, S. Regulation of STAT Signaling by Acetylation. Cell. Signal. 2013, 25, 1924–1931. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-ΚB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- In, H.L.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A Role for the NAD-Dependent Deacetylase Sirt1 in the Regulation of Autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol Improves Oxidative Stress and Protects against Diabetic Nephropathy through Normalization of Mn-SOD Dysfunction in AMPK/SIRT1- Independent Pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef]
- Xu, Y.; Nie, L.; Yin, Y.G.; Tang, J.L.; Zhou, J.Y.; Li, D.D.; Zhou, S.W. Resveratrol Protects against Hyperglycemia-Induced Oxidative Damage to Mitochondria by Activating SIRT1 in Rat Mesangial Cells. Toxicol. Appl. Pharmacol. 2012, 259, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, H.; Zhou, W.; Luo, M.; Tan, Y.; Miao, L.; Cai, L. Sirtuin 1: A Target for Kidney Diseases. Mol. Med. 2015, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, S.; Gao, S.; Wang, Y.; Cai, X.; Fang, J.; Yan, T.; Wan, C.; Cai, Y. Sirtuins: Research Advances on the Therapeutic Role in Acute Kidney Injury. Phytomedicine 2022, 101, 154122. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Huang, X.; Zhang, M.; Zhang, L.; Chen, J.; Gu, Y.; Hao, C.M. Resveratrol Attenuates Diabetic Nephropathy via Modulating Angiogenesis. PLoS ONE 2013, 8, e82336. [Google Scholar] [CrossRef]
- Qiu, D.; Song, S.; Wang, Y.; Bian, Y.; Wu, M.; Wu, H.; Shi, Y.; Duan, H. NAD(P)H: Quinone Oxidoreductase 1 Attenuates Oxidative Stress and Apoptosis by Regulating Sirt1 in Diabetic Nephropathy. J. Transl. Med. 2022, 20, 44. [Google Scholar] [CrossRef]
- Gong, H.; Zheng, C.; Lyu, X.; Dong, L.; Tan, S.; Zhang, X. Inhibition of Sirt2 Alleviates Fibroblasts Activation and Pulmonary Fibrosis via Smad2/3 Pathway. Front. Pharmacol. 2021, 12, 756131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, X.; Ruan, X.; Wei, Q.; Zhang, L.; Wo, L.; Huang, D.; Lin, L.; Wang, D.; Xia, L.; et al. SIRT2-Mediated Deacetylation and Deubiquitination of C/EBPβ Prevents Ethanol-Induced Liver Injury. Cell Discov. 2021, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Alt, F.W.; Cheng, H.-L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective Effects of Sirtuin 3 in a Murine Model of Sepsis-Induced Acute Kidney Injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, A.; Gali, S.; Sharma, S.; Park, J.H.; Kyung, S.Y.; Kacew, S.; Kim, I.S.; Lee, K.Y.; Kim, H.S. Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways. Antioxidants 2022, 11, 1812. https://doi.org/10.3390/antiox11091812
Kundu A, Gali S, Sharma S, Park JH, Kyung SY, Kacew S, Kim IS, Lee KY, Kim HS. Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways. Antioxidants. 2022; 11(9):1812. https://doi.org/10.3390/antiox11091812
Chicago/Turabian StyleKundu, Amit, Sreevarsha Gali, Swati Sharma, Jae Hyeon Park, So Young Kyung, Sam Kacew, In Su Kim, Kwang Youl Lee, and Hyung Sik Kim. 2022. "Tenovin-1 Ameliorates Renal Fibrosis in High-Fat-Diet-Induced Diabetic Nephropathy via Antioxidant and Anti-Inflammatory Pathways" Antioxidants 11, no. 9: 1812. https://doi.org/10.3390/antiox11091812