1. Introduction
Indonesia is one of the world’s leading producers of mangosteen fruit (
Garcinia mangostana L.), both fresh and processed into canned products and juices [
1,
2]. During mangosteen processing, the peel is separated and discarded as industrial waste, even though it offers numerous benefits. Mangosteen peel can be used as a food, supplement, cosmetic, and medicine because it contains many therapeutic compounds and antioxidants [
3,
4,
5,
6,
7,
8,
9]. Mangosteen peel is rarely ingested raw due to its astringent taste. The major antioxidant components in mangosteen peel, xanthones, have poor water solubility, and direct usage reduces their efficiency since they can be degraded or oxidized during processing and storage. According to reports, the microencapsulation technique can overcome these challenges [
10,
11,
12].
Microencapsulation is the process of encapsulating tiny solid, liquid, or gaseous particles in a polymeric substance. The polymer material can modulate odor, taste, volatility, and reactivity, as well as enhance the extract material’s stability and solubility [
13,
14,
15,
16]. Many factors affect the quality of microcapsules, including the preparation technique and the type of coating material [
17]. Fluidized bed microencapsulation is the prevalent approach and offers several advantages, including low cost, minimal dust pollution, high uniformity, high encapsulation efficiency, and excellent stability [
18,
19]. The polymer utilized further enhances the quality and stability of the microencapsulation; hence, choosing the proper base polymer should be performed [
17,
20].
Several natural polymers have been used as core and coating materials in microencapsulation. One of them is HPMC, which is known to be biodegradable, biocompatible, renewable, and low in toxicity [
21,
22]. HPMC has been used as a mixture with active ingredients to increase solubility and extend drug contact time due to its hydrophilic and mucoadhesive properties [
23,
24,
25]. This cellulose derivate has also demonstrated substantial potential to alleviate GI irritation caused by APIs known to induce diarrhea, ulcers, nausea, or vomiting [
23]. Since HPMC is hygroscopic, particularly after drying, it is typically mixed with other polymers [
26,
27] or coated [
23]. As a coating material, PVA offers several advantages, including biodegradability, biocompatibility, and good mechanical properties [
28]. The PVA film is both water- and heat-resistant, which contributes to the core material’s stability [
29]. The study by Wu et al. reveals that employing PVA as a coating can minimize the size of the microencapsulation while also providing protection against coalescence [
30]. The encapsulation efficiency of PVA is also reported to be high [
31]. As a result, we employed HPMC as the core material’s binder and PVP as the microspheres’ coating. In this work, we investigated the stability and antioxidant activity of mangosteen peel extract during its transformation into microcapsules and, lastly, tablets. The characteristics of microcapsules, granules, and tablets were also studied.
2. Materials and Methods
2.1. Materials
We used the dry mangosteen peel powder obtained from Subang, Indonesia, ethanol 96% (PT. Brataco, Indonesia), polyvinyl alcohol (PVA) (Sigma Aldrich, St. Louis, MO, USA), polyvinylpyrrolidone (PVP K30) (Sigma Aldrich, St. Louis, MO, USA), citric acid (PT. Brataco, Indonesia), sodium citrate (PT. Brataco, Indonesia), xanthan gum (Sigma Aldrich, St. Louis, MO, USA), sucrose (PT. Brataco, Indonesia), carboxymethylcellulose sodium (Sigma Aldrich, St. Louis, MO, USA), hydroxypropylmethylcellulose (HPMC) (Sigma Aldrich, St. Louis, MO, USA), 1,1-diphenyl-2-picryl-hydrazyl (DPPH) (Sigma Aldrich, St. Louis, MO, USA), Mg stearate (Sigma Aldrich, St. Louis, MO, USA), Amprotab (Sigma Aldrich, St. Louis, MO, USA), talcum (Sigma Aldrich, St. Louis, MO, USA), and lactose (PT. Brataco, Indonesia).
2.2. Mangosteen Peel Extraction
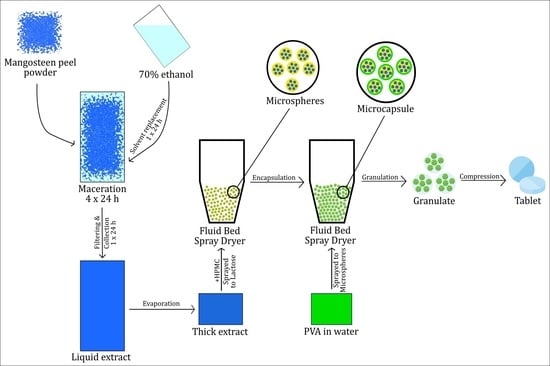
The 25 kg of fine dry mangosteen peel powder was placed in the macerator (Extraction and Concentration Machine TD-300, China) and soaked in aqueous ethanol solution 70% (v/v) until all the powder was wetted, then ethanol was added again until the powder was completely submerged. This procedure was carried out for 4 days, with a replacement of solvent every 24 h. Each macerate was filtered and mixed before being evaporated to make a thick extract in a rotary evaporator (BUCHI Rotavapor R-300, PT. BUCHI, Indonesia) at 40 °C and 30 rpm.
2.3. Phytochemical Screening, Standardization, Thin-Layer Chromatography Profile, and Antioxidant Activity of Mangosteen Peel Extract
Phytochemical screening was conducted to determine the secondary metabolite content of mangosteen peel extract (MPE). The phytochemical screening included alkaloids, tannins, polyphenols, flavonoids, quinones, saponins, monoterpenes, sesquiterpenes, steroids, and triterpenoids. Standardization was also carried out to determine the quality of MPE. Types of inspection performed were organoleptic, ethanol-soluble extract content, water-soluble extract content, total ash content, acid-insoluble ash content, and drying shrinkage.
The thin-layer chromatography profile of MPE was examined using a silica plate GF 254 with a mixed mobile phase of chloroform and ethyl acetate (9:1). After the chamber was saturated, 5% v/v MPE solution in methanol was applied to the starting line on the silica plate and then waited for the mobile phase to reach the finish line on the silica plate. The retention factor value of each spot was recorded.
The antioxidant activity of MPE was determined using the DPPH reagent. The sample and positive control (ascorbic acid) were prepared to as much as 100 µL with a concentration range of 10–50 and 1–5 ppm, respectively. A total of 0.1 mL of DPPH reagent (0.2 mg/mL in ethanol) was added to the sample and the positive control. The sample and positive control were allowed to stand for 30 min in the dark (25 °C), after which the absorbance was measured at a wavelength of 517 nm (Epoch™ Microplate Spectrophotometer, BioTek Instrument, Inc., Winooski, VT, USA). The IC50 value was calculated from the linear equation of %inhibition vs. concentrations, where %inhibition was obtained using the following equation:
2.4. Total Phenolic and α-Mangosteen Content of Mangosteen Peel Extract
Total phenolic of MPE was determined using gallic acid as the standard. The standard was made into several variations of concentration (200–600 ppm). The extract solution was prepared by dissolving 10 mg of MPE in 10 mL of methanol. One mL of the extract solution and each concentration of the standard solution was put into a dilution tube, then 5 mL of Folin–Ciocalteau reagent was added. The preparations were mixed for 8 min, then 4 mL of 1% v/v NaOH was added. The absorbance of the extract solution and the standard were measured at a wavelength of 256 nm (Epoch™ Microplate Spectrophotometer, BioTek Instrument, Inc., Winooski, VT, USA).
Determination of the α-mangosteen content was carried out using high-performance liquid chromatography (HPLC) (Waters Alliance HPLC, Waters Corporation, USA). The standard solution of α-mangosteen was made into several concentrations in the range of 10–50 ppm to create the standard curve. The extract solution (10 mg/mL in methanol) was filtered using a syringe filter and then inserted into the HPLC sample tube. The column used was C18. The mobile phase used was methanol and aquades (95:5 v/v). The injection volume was set to 10 L with a flow rate of 1 mL/min. The retention time of α-mangosteen was 10 min and was measured at a wavelength of 318 nm using a UV detector.
2.5. Mangosteen Peel Extract Microencapsulation
Microcapsules of MPE (MPEM) were prepared using a Fluid Bed Spray Dryer (PMS FBD5, Armitec, Thailand). Outlet temperature was set at 37.2 °C, with a spray interval of 10 s, product temperature of 37.8 °C, inlet temperature of 37.8 °C, and spray rate of 20.5 rpm. The core material (microspheres) was prepared by spraying a mixture of 20% w/v HPMC and 33.3% v/v MPE (600 mL) onto 1.5 kg of lactose. After the mixture was dry and homogeneous, the microspheres were again sprayed with 15% w/v polyvinyl alcohol in 450 mL of water as a coating agent. The thin-layer chromatography profile and IC50 of MPEM were then determined using the previously described method.
2.6. Characterization of Mangosteen Peel Extract Microcapsule
2.6.1. Moisture Content
A total of 1 g of MPEM was placed in a dish on a moisture balance device (Moisture Analyzer MA 50.R, Radwag, Miami, FL, USA). The temperature was set at 105 °C. Drying loss was recorded after the tool showed constant weight during heating.
2.6.2. Flow Rate and Angle of Repose
A total of 25 g of MPEM was placed in the funnel of the flowmeter (GTB Series, Erweka, Langen, Germany). The MPEM flow rate was determined by observing the time it takes for MPEM to pass through the funnel until it runs out. The angle of repose was obtained by measuring the diameter and height of the MPEM pile formed.
2.6.3. Compressibility Index
A total of 25 g of MPEM was put into a measuring cup contained in the volumenometer (Tapped Density Tester, Erweka SVM 221, Erweka). The compressibility index of MPEM was determined by the final volume of the microcapsule after 500 beats.
2.6.4. Shape, Morphology, and Particle Size
The shape and surface morphology of MPEM were observed using a scanning electron microscope (SEM) (JSM-6360, Jeol, Tokyo, Japan) with 500× magnification. The particle size of MPEM was then determined using a particle size analyzer (Horiba SZ-100, Horiba Ltd., Kyoto, Japan). The test was carried out by dispersing the sample in a phosphate buffer at pH 6.8, after which a 1 mL sample was taken for testing.
2.6.5. Solubility or Sedimentation Volume
A total of 5 g of MPEM was dissolved in 100 mL of distilled water and then stirred for 20 s. Sedimentation volume was measured after 15 min.
2.7. Tablet Formulation and Evaluation
MPEM tablets were made by mixing MPEM and internal phase materials (binder, disintegrant, and filler). The wet granulation method was used for the granulation. After the granules were formed, the external phase (lubricant, glidant, and filler residue) was added to the granule mass. The optimal binder was chosen using a formula optimization method, as indicated in
Table 1. The amount of MPEM used was 435 mg, equivalent to 30 mg of ascorbic acid (based on the IC50 value). The granule mass was then molded with a diameter of 0.9 cm and a thickness of 0.33 cm. The total weight per tablet was 750 mg.
The characteristics of the granules and tablets were evaluated to determine the quality of the tablets in each test formula. The thin-layer chromatography profile and IC50 of the best formulas were determined using the previously described method. The examined granules’ properties were drying shrinkage, flow rate, angle of repose, and compressibility index using the same method for evaluating the quality of the microcapsules. The tablets evaluations were as follows.
2.7.1. Organoleptic
Organoleptic examination of MPEM tablets was carried out by observing the shape, color, and aroma of the 10 whole tablets. To assess the taste, the tablet was crushed into a powder and a certain amount of the powder was then tasted. This test was assessed by 8 people of the research project team.
2.7.2. Hardness Test
A hardness test was performed by placing the tablet on the hardness tester (Monsato VMT, Vinsyst Technologies, Mumbai, India). The hardness tester applied increasing pressure periodically until the tablet cracked. The tablet hardness was then recorded in the N unit. The number of samples used in this procedure was 10 tablets.
2.7.3. Size and Weight Uniformity
A total of 20 tablets were prepared to check the uniformity of the tablets’ size and weight. Tablet dimension (diameter and thickness) was measured using a caliper (Vernier Caliper, Tricle Brand, Shanghai, China), while the tablet weight was weighed using an analytical balance (Mettler Toledo ME204, PT. Mettler-Toledo, Bekasi City, Indonesia). Average dimensions and weight were then calculated along with the standard deviation.
2.7.4. Friability Test
The friability test was carried out by placing 20 weighed tablets into the friability tester (Biobase TFT-2, Biobase Group, Jinan, China). The rotational speed of the friabilator was set at 400 rpm for 15 min. After that, the tablets were removed and reweighed to obtain the average weight.
2.7.5. Disintegration Test
The disintegration test was carried out using a disintegration tester (Disintegration Tester TDT-2IM, Zhengzhou, China) with a water medium at 37 ± 2 °C. Disintegration time was recorded when the tablets in the basket were completely crushed.
2.8. Statistical Analysis
The paired T-test method was used to assess the significance of the change in antioxidant activity (IC50) in each intermediate product. The data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) version 22 (IBM Corporation, New York, NY, USA).