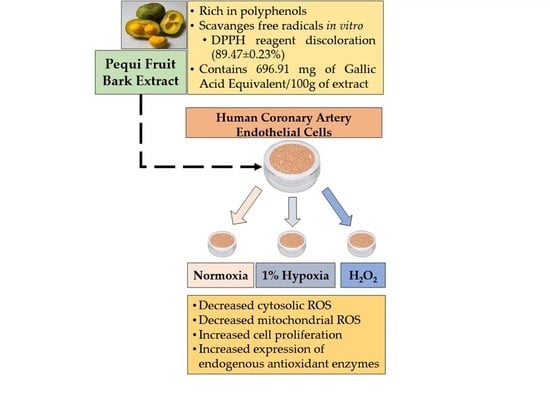
Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pequi Extract Preparation
2.2. Total Phenol Quantification and Antioxidant Activity In Vitro of Pequi Extract
2.3. Qualitative High-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (HPLC-HRMS)
2.4. Cell Culture
2.5. Stress Induction in HCAEC
2.6. Determination of Cytosolic ROS Production in HCAEC
2.7. Determination of Mitochondrial ROS Production in HCAEC
2.8. Proliferation Assay in HCAEC Subjected to Hypoxia and H2O2
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Pequi Extract Is Rich in Phenolic Compounds
3.2. Pequi Extract Scavenges ROS In Vitro
3.3. Pequi Extract Can Reduce Cytosolic and Mitochondrial ROS Levels in HCAEC
3.4. Pequi Extract Increases Proliferation in HCAEC
3.5. Pequi Extract Induces Expression of Antioxidant Enzymes in HCAEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heron, M. Deaths: Leading Causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 1–76. [Google Scholar] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Bailão, E.F.L.C.; Devilla, I.A.; da Conceição, E.C.; Borges, L.L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Willcox, B.J.; Curb, J.D.; Rodriguez, B.L. Antioxidants in Cardiovascular Health and Disease: Key Lessons from Epidemiologic Studies. Am. J. Cardiol. 2008, 101, S75–S86. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Lichtenstein, A.H.; Howard, B.V.; Steinberg, D.; Witztum, J.L. Nutrition Committee of the American Heart Association Council on Nutrition, Physical Activity, and Metabolism Antioxidant Vitamin Supplements and Cardiovascular Disease. Circulation 2004, 110, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Feher, A.; Beleznai, T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: Implications for reactive oxygen species. Pharmacol. Rep. 2009, 61, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Li, J.; Yuan, Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Holanda, R.B.; Sousa, C.A.S.; Pastore, G.M. Atividade antioxidante de frutas do cerrado. Ciência Tecnol. Aliment. 2007, 27, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.C.L.; Morais, L.M.O.; Guimarães, A.Q.; Almada, E.D.; Barbosa, P.M.; Drumond, M.A. Traditional knowledge and uses of the Caryocar brasiliense Cambess. (Pequi) by “quilombolas” of Minas Gerais, Brazil: Subsidies for sustainable management. Braz. J. Biol. 2016, 76, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares Júnior, M.S.; Bassinello, P.Z.; Caliari, M.; dos Reis, R.C.; Lacerda, D.B.C.L.; Koakuzu, S.N. Development and chemical characterization of flour obtained from the external mesocarp of “pequizeiro” fruit. Ciência Tecnol. Aliment. 2010, 30, 940–948. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, L.S.; Pereira, K.F.; de Araújo, E.G. Botanical features, therapeutic effects and active ingredients present in pequi (Caryocar brasiliense). Arq. Ciências Saúde UNIPAR 2015, 19, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Jorge, A.; Leitão, M.M.; Bernal, L.P.T.; Dos Santos, E.; Kuraoka-Oliveira, Â.M.; Justi, P.; Argandoña, E.; Kassuya, C.A.L. Analgesic and anti-inflammatory effects of Caryocar brasiliense. Antiinflamm. Antiallergy Agents Med. Chem. 2019, 18, 313–322. [Google Scholar] [CrossRef]
- Roll, M.M.; Miranda-Vilela, A.L.; Longo, J.P.F.; da Agostini-Costa, T.S.; Grisolia, C.K. The pequi pulp oil (Caryocar brasiliense Camb.) provides protection against aging-related anemia, inflammation and oxidative stress in Swiss mice, especially in females. Genet. Mol. Biol. 2018, 41, 858–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, T.S.; Thomaz, D.V.; da Silva Neri, H.F.; Cerqueira, L.B.; Garcia, L.F.; Gil, H.P.V.; Pontarolo, R.; Campos, F.R.; Costa, E.A.; Dos Santos, F.C.A.; et al. Neuroprotective effect of caryocar brasiliense camb. leaves is associated with anticholinesterase and antioxidant properties. Oxid. Med. Cell. Longev. 2018, 2018, 9842908. [Google Scholar] [CrossRef] [Green Version]
- Roesler, R.; Lorencini, M.; Pastore, G. Fontes de antioxidantes do cerrado brasileiro: Citotoxicidade e fototoxicidade in vitro. Cienc. Tecnol. Aliment. 2010, 30, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.R.; de Carvalho, R.K.; de Carvalho, L.S.; de Sá, S.; Andersen, M.L.; de Araújo, E.G.; Mazaro-Costa, R. Effects of subchronic exposure to Caryocar brasiliense peel ethanolic extract on male reproductive functions in Swiss mice. Reprod. Toxicol. 2019, 87, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Volume 6, pp. I1.1.1–I1.1.8. [Google Scholar]
- Wu, D.; Yotnda, P. Induction and testing of hypoxia in cell culture. J. Vis. Exp. 2011, 54, e2899. [Google Scholar] [CrossRef] [Green Version]
- Shafique, E.; Torina, A.; Reichert, K.; Colantuono, B.; Nur, N.; Zeeshan, K.; Ravichandran, V.; Liu, Y.; Feng, J.; Zeeshan, K.; et al. Mitochondrial redox plays a critical role in the paradoxical effects of NAPDH oxidase-derived ROS on coronary endothelium. Cardiovasc. Res. 2017, 113, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, Y.; Pan, R.L.; Wang, R.Y.; Ding, S.L.; Dong, W.R.; Sun, G.B.; Ye, J.X.; Sun, X.B. Protective effects of Myrica rubra flavonoids against hypoxia/reoxygenation-induced cardiomyocyte injury via the regulation of the PI3K/Akt/GSK3β pathway. Int. J. Mol. Med. 2019, 43, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Alam, M.B.; Ju, M.-K.; Kwon, K.-R.; Huh, Y.S.; Han, Y.-K.; Lee, S.H. Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomed. Pharmacother. 2018, 103, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.; Nevárez-Moorillón, G.V.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant activity of Caryocar brasiliense (pequi) and characterization of components by electrospray ionization mass spectrometry. Food Chem. 2008, 110, 711–717. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H. Bin Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genomics 2018, 50, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, L.; Chen, W.; Xu, S.; Feng, X.; Zhang, L. Natural products: The role and mechanism in low-density lipoprotein oxidation and atherosclerosis. Phyther. Res. 2021, 35, 2945–2967. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A. Polyphenols Effect on Circulating Lipids and Lipoproteins: From Biochemistry to Clinical Evidence. Curr. Pharm. Des. 2018, 24, 178–190. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, P.R.L.; Oliveira, I.B.; Neto, J.B.S.; de Oliveira, J.A.; Ribeiro, L.B.; de Barros Viana, G.S.; Rocha, T.M.; Leal, L.K.A.M.; Kerntopf, M.R.; Felipe, C.F.B.; et al. Caryocar coriaceum Wittm. (Pequi) fixed oil presents hypolipemic and anti-inflammatory effects in vivo and in vitro. J. Ethnopharmacol. 2016, 191, 87–94. [Google Scholar] [CrossRef]
- Silva, G.T.; Di Pietro Fernandes, C.; Hiane, P.A.; De Cássia Freitas, K.; Figueiredo, P.S.; Inada, A.C.; Filiú, W.F.; Maldonade, I.R.; Nunes, Â.A.; De Oliveira, L.C.S.; et al. Caryocar brasiliense cambess. pulp oil supplementation reduces total cholesterol, LDL-c, and Non-HDL-c in animals. Molecules 2020, 25, 4530. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Chen, S.-C.; Lin, K.-Y.; Wang, M.-T.; Chen, Y.-C.; Huang, H.-C.; Cho, H.-J.; Wang, L.; Kumar, K.J.S.; Hseu, Y.-C. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J. Ethnopharmacol. 2011, 137, 669–680. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Mohammadi, S. Important signals regulating coronary artery angiogenesis. Microvasc. Res. 2018, 117, 1–9. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, K.M.S.; Araujo, E.G.; Sellke, F.W.; Abid, M.R. Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells. Antioxidants 2022, 11, 474. https://doi.org/10.3390/antiox11030474
Braga KMS, Araujo EG, Sellke FW, Abid MR. Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells. Antioxidants. 2022; 11(3):474. https://doi.org/10.3390/antiox11030474
Chicago/Turabian StyleBraga, Karla M. S., Eugenio G. Araujo, Frank W. Sellke, and M. Ruhul Abid. 2022. "Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells" Antioxidants 11, no. 3: 474. https://doi.org/10.3390/antiox11030474