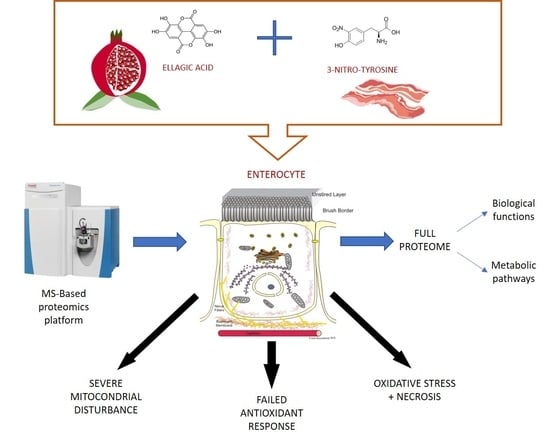
Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Chemicals
2.2. Cell Culture
2.3. Experimental Setting
2.4. Flow Cytometry Analyses
2.5. Quantification of Protein Carbonyls
2.6. Endogenous Antioxidant Enzymes
2.7. Sample Preparation for LC-MS/MS Based Proteomics
2.8. Label-Free Quantitative Proteomic Analyses
2.9. Statistical Analysis
3. Results
3.1. Flow Cytometry and Protein Oxidation Markers
3.2. Proteomic Analyses
3.2.1. Proteins Found in Lower Relative Quantity in Cells Exposed to 3-NT Compared to Control Counterparts
Antigen Processing and Presentation of Exogenous Peptide Antigen via MHC Class II
Cytoplasmic Translational Initiation
Nucleosome Assembly
Mitotic Cytokinesis
Intramolecular Oxidoreductase Activity, Transposing C=C bonds
Tau Protein Binding
Other Proteins of Biological Significance Found in Lower Relative Quantity in 3-NT-Treated Cells
3.2.2. Proteins Found in Higher Relative Quantity in Cells Exposed to 3-NT Compared to Control Counterparts
Positive Regulation of Cyclic-Nucleotide Phosphodiesterase Activity
Type 3 Metabotropic Glutamate Receptor Binding
Other Proteins of Biological Significance Found in Higher Relative Quantity in 3-NT-Treated Cells
3.2.3. Proteins Found in Lower Relative Quantity in Cells Exposed to 3-NT+EA Compared to 3-NT Counterparts
Intracellular Transport
Purine Nucleotide Metabolic Process
Nucleotide Binding and Nitric Oxide Synthase Regulator Activity
3.2.4. Proteins Found in Higher Relative Quantity in Cells Exposed to 3-NT+EA Compared to 3-NT Counterparts
Posttranscriptional Regulation of Gene Expression
Organic Substance Catabolic Process
Protein-Containing Complex Assembly
Oxidoreductase Activity
Aminopeptidase Activity
Oxidoreductase Activity, Acting on a Sulfur Group of Donors
Other Proteins of Biological Significance Found in Higher Relative Quantity
3.3. Endogenous Antioxidant Enzyme Activity
4. Discussion
4.1. Impact of 3-NT and 3-NT+EA on Calmodulin-Dependent Intracellular Signaling Pathway
4.2. Impact of 3-NT and 3-NT+EA on Immune System and Steroid Hormones
4.3. Impact of 3-NT and 3-NT+EA on Antioxidant Defenses and Oxidative Stress
4.4. Impact of 3-NT and 3-NT+EA in Cell Viability, Apoptosis, Necrosis and Protein Repair
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estévez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Health Risks of Food Oxidation. Adv. Food Nutr. Res. 2017, 82, 45–81. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.; Ventanas, J.; Estévez, M. Nitrite Promotes Protein Carbonylation and Strecker Aldehyde Formation in Experimental Fermented Sausages: Are Both Events Connected? Meat Sci. 2014, 98, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, V.H.; Otles, S. Investigation of the Effect of Sodium Nitrite on Protein Oxidation Markers in Food Protein Suspensions. J. Food Biochem. 2020, 44, e13152. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Pöschl, U. Quantification of Nitrotyrosine in Nitrated Proteins. Anal. Bioanal. Chem. 2010, 397, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard-Fillion, B.; Prou, D.; Polydoro, M.; Spielberg, D.; Tsika, E.; Wang, Z.; Hazen, S.L.; Koval, M.; Przedborski, S.; Ischiropoulos, H. Metabolism of 3-Nitrotyrosine Induces Apoptotic Death in Dopaminergic Cells. J. Neurosci. 2006, 26, 6124–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.L.; Wei, J.R. 3-Nitrotyrosine, a Biomarker for Cardiomyocyte Apoptosis Induced by Diabetic Cardiomyopathy in a Rat Model. Mol. Med. Rep. 2013, 8, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Velasco, S.; González, A.; Peña, F.J.; Estévez, M. Noxious Effects of Selected Food-Occurring Oxidized Amino Acids on Differentiated CACO-2 Intestinal Human Cells. Food Chem. Toxicol. 2020, 144, 111650. [Google Scholar] [CrossRef]
- Villaverde, A.; Morcuende, D.; Estévez, M. Effect of Curing Agents on the Oxidative and Nitrosative Damage to Meat Proteins during Processing of Fermented Sausages. J. Food Sci. 2014, 79, C1331–C1342. [Google Scholar] [CrossRef]
- Vossen, E.; De Smet, S. Protein Oxidation and Protein Nitration Influenced by Sodium Nitrite in Two Different Meat Model Systems. J. Agric. Food Chem. 2015, 63, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- IARC. Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheshomi, H.; Bahrami, A.R.; Matin, M.M. Ellagic Acid and Human Cancers: A Systems Pharmacology and Docking Study to Identify Principal Hub Genes and Main Mechanisms of Action. Mol. Divers. 2021, 25, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Ellagic Acid in Suppressing in Vivo and in Vitro Oxidative Stresses. Mol. Cell. Biochem. 2018, 448, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Velasco, S.; Delgado, J.; Peña, F.J.; Estévez, M. Protein Oxidation Marker, α-Amino Adipic Acid, Impairs Proteome of Differentiated Human Enterocytes: Underlying Toxicological Mechanisms. Biochim. Biophys. Acta Proteins Proteom. 2022, 1870, 140797. [Google Scholar] [CrossRef]
- Pinto, M.; Robine Leon, S.; Appay, M.D. Enterocyte-like Differentiation and Polarization of the Human Colon Carcinoma Cell Line Caco-2 in Culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Utrera, M.; Morcuende, D.; Rodríguez-Carpena, J.G.; Estévez, M. Fluorescent HPLC for the Detection of Specific Protein Oxidation Carbonyls—α-Aminoadipic and γ-Glutamic Semialdehydes—In Meat Systems. Meat Sci. 2011, 89, 500–506. [Google Scholar] [CrossRef]
- Akagawa, M.; Sasaki, T.; Suyama, K. Oxidative Deamination of Lysine Residue in Plasma Protein of Diabetic Rats: Novel Mechanism via the Maillard Reaction. Eur. J. Biochem. 2002, 269, 5451–5458. [Google Scholar] [CrossRef]
- Delgado, J.; Núñez, F.; Asensio, M.A.; Owens, R.A. Quantitative Proteomic Profiling of Ochratoxin A Repression in Penicillium Nordicum by Protective Cultures. Int. J. Food Microbiol. 2019, 305, 108243. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Estévez, M.; Xiong, Y. Intake of Oxidized Proteins and Amino Acids and Causative Oxidative Stress and Disease: Recent Scientific Evidences and Hypotheses. J. Food Sci. 2019, 84, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Vorherr, T.; Knöpfel, L.; Hofmann, F.; Carafoli, E.; Mollner, S.; Pfeuffer, T. The Calmodulin Binding Domain of Nitric Oxide Synthase and Adenylyl Cyclase. Biochemistry 1993, 32, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.W.; Olmo, R.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptors and Neurodegenerative Diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kammer, G.M. The Adenylate Cyclase-CAMP-Protein Kinase A Pathway and Regulation of the Immune Response. Immunol. Today 1988, 9, 222–229. [Google Scholar] [CrossRef]
- London, E.; Bloyd, M.; Stratakis, C.A. PKA Functions in Metabolism and Resistance to Obesity: Lessons from Mouse and Human Studies. J. Endocrinol. 2020, 246, R51–R64. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine Receptors: Structure, Expression, Molecular Details, and Function in Calcium Release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [Green Version]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular Calcium Release Channels: An Update. J. Physiol. 2017, 595, 3041. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, K.E.; Novak, E.A.; Vincent, G.; Siow, V.S.; Griffith, B.D.; Ranganathan, S.; Rosengart, M.R.; Piganelli, J.D.; Mollen, K.P. Calcium/Calmodulin–Dependent Protein Kinase IV (CaMKIV) Activation Contributes to the Pathogenesis of Experimental Colitis via Inhibition of Intestinal Epithelial Cell Proliferation. FASEB J. 2019, 33, 1330–1346. [Google Scholar] [CrossRef]
- Dubey, R.K.; Gillespie, D.G.; Jackson, E.K. Cyclic AMP-Adenosine Pathway Induces Nitric Oxide Synthesis in Aortic Smooth Muscle Cells. Hypertension 1998, 31, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.; Naviliat, M.; Tiscornia, A.C.; Vuillier, F.; Gualco, G.; Dighiero, G.; Radi, R.; Cayota, A.M. Peroxynitrite Inhibits T Lymphocyte Activation and Proliferation by Promoting Impairment of Tyrosine Phosphorylation and Peroxynitrite-Driven Apoptotic Death. Am. Assoc. Immnol. 1999, 162, 3356–3366. [Google Scholar]
- Birnboim, H.C.; Lemay, A.-M.; Lam, D.K.Y.; Goldstein, R.; Webb, J.R. Cutting Edge: MHC Class II-Restricted Peptides Containing the Inflammation-Associated Marker 3-Nitrotyrosine Evade Central Tolerance and Elicit a Robust Cell-Mediated Immune Response. J. Immunol. 2003, 171, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Udenwobele, D.I.; Su, R.C.; Good, S.V.; Ball, T.B.; Shrivastav, S.V.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Heidarian, F.; Kazemi Oskuee, R. Ellagic Acid as a Potent Anticancer Drug: A Comprehensive Review on in Vitro, in Vivo, in Silico, and Drug Delivery Studies. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Wosen, J.E.; Mukhopadhyay, D.; MacAubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Betapudi, V. Myosin II Motor Proteins with Different Functions Determine the Fate of Lamellipodia Extension during Cell Spreading. PLoS ONE 2010, 5, e8560. [Google Scholar] [CrossRef] [Green Version]
- Sebbagh, M.; Hamelin, J.; Bertoglio, J.; Solary, E.; Bréard, J. Direct Cleavage of ROCK II by Granzyme B Induces Target Cell Membrane Blebbing in a Caspase-Independent Manner. J. Exp. Med. 2005, 201, 465–471. [Google Scholar] [CrossRef]
- Ma, M.P.C.; Chircop, M. SNX9, SNX18 and SNX33 Are Required for Progression through and Completion of Mitosis. J. Cell Sci. 2012, 125 Pt 18, 4372–4382. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.Y.; Kranzusch, P.J.; Doudna, J.A.; Cate, J.H.D. EIF3d Is an MRNA Cap-Binding Protein That Is Required for Specialized Translation Initiation. Nature 2016, 536, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Ayloo, S.; Lazarus, J.E.; Dodda, A.; Tokito, M.; Ostap, E.M.; Holzbaur, E.L.F. Dynactin Functions as Both a Dynamic Tether and Brake during Dynein-Driven Motility. Nat. Commun. 2014, 5, 4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Shu, C.; Gao, X.; Sankaran, B.; Du, F.; Shelton, C.L.; Herr, A.B.; Ji, J.Y.; Li, P. Structural Basis for Concerted Recruitment and Activation of IRF-3 by Innate Immune Adaptor Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E3403–E3412. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Corona, N.C.; Lopez-Ortega, O.; Flores Hermenegildo, J.M.; Berron-Ruiz, L.; Rodriguez-Alba, J.C.; Santos-Argumedo, L.; Lopez-Herrera, G. Lipopolysaccharide-Responsive Beige-like Anchor Acts as a CAMP-Dependent Protein Kinase Anchoring Protein in B Cells. Scand. J. Immunol. 2020, 92, e12922. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A Biomarker of Nitrogen Free Radical Species Modified Proteins in Systemic Autoimmunogenic Conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Fernandes, R.; Prudêncio, C.; Vieira, M. 3-Nitrotyrosine Quantification Methods: Current Concepts and Future Challenges. Biochimie 2016, 125, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lachance, Y.; Luu-the, V.; Verreault, H.; Dumont, M.; Rhéaume, E.; Leblanc, G.; Labrie, F. Structure of the Human Type II 3 Beta-Hydroxysteroid Dehydrogenase/Delta 5-Delta 4 Isomerase (3 Beta-HSD) Gene: Adrenal and Gonadal Specificity. DNA Cell Biol. 1991, 10, 701–711. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Steroid Hormone Action. Yen Jaffes Reprod. Endocrinol. Physiol. Pathophysiol. Clin. Manag. Eighth Ed. 2019, 115–131. [Google Scholar] [CrossRef]
- Atanassova, N.; Koeva, Y. Hydrohysteroid Dehydrogenases—Biological Role and Clinical Importance—Review. Dehydrogenases 2012. [Google Scholar] [CrossRef] [Green Version]
- Requena, J.R.; Chao, C.C.; Levine, R.L.; Stadtman, E.R. Glutamic and Aminoadipic Semialdehydes Are the Main Carbonyl Products of Metal-Catalyzed Oxidation of Proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 69–74. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, S.; Harner, M.E. The MICOS Complex, a Structural Element of Mitochondria with Versatile Functions. Biol. Chem. 2020, 401, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Gao, H.; Yang, D.; He, X.; Fang, Y.; Zhou, G. Phenolic Compound Ellagic Acid Inhibits Mitochondrial Respiration and Tumor Growth in Lung Cancer. Food Funct. 2020, 11, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Shafie, B.; Pourahmad, J.; Rezaei, M. N-Acetylcysteine Is More Effective than Ellagic Acid in Preventing Acrolein Induced Dysfunction in Mitochondria Isolated from Rat Liver. J. Food Biochem. 2021, 45, e13775. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Padilla, L.; Romeu, X.; Roca, J.; Barranco, I.; Yeste, M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs—Relationship with Sperm Morphology. Antioxidants 2020, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Schaich, K.M. Toxicity of Lipid Oxidation Products Consumed in the Diet. Baileys Ind. Oil Fat Prod. 2020, 1–88. [Google Scholar] [CrossRef]
- Davis, C.W.; Hawkins, B.J.; Ramasamy, S.; Irrinki, K.M.; Cameron, B.A.; Islam, K.; Daswani, V.P.; Doonan, P.J.; Manevich, Y.; Madesh, M. Nitration of the Mitochondrial Complex I Subunit NDUFB8 Elicits RIP1- and RIP3-Mediated Necrosis. Free Radic. Biol. Med. 2010, 48, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Ott, C.; Dorsch, E.; Fraunholz, M.; Straub, S.; Kozjak-Pavlovic, V. Detailed Analysis of the Human Mitochondrial Contact Site Complex Indicate a Hierarchy of Subunits. PLoS ONE 2015, 10, e0120213. [Google Scholar] [CrossRef]
- Bragança, C.E.; Kraut, D.A. Mode of Targeting to the Proteasome Determines GFP Fate. J. Biol. Chem. 2020, 295, 15892–15901. [Google Scholar] [CrossRef]
- Hass, C.S.; Lam, K.; Wold, M.S. Repair-Specific Functions of Replication Protein A. J. Biol. Chem. 2012, 287, 3908–3918. [Google Scholar] [CrossRef] [PubMed]




| Protein Name | Gene Name | p-Value | Fold-Change 1 | Biological Function | FASTA Accession Number |
|---|---|---|---|---|---|
| Rho-associated protein kinase 2 | ROCK2 | - | C | Involved in blebs formation | O75116 |
| STAM-binding protein | STAMBP | - | C | Associated with intracellular accumulation of ubiquitinated protein | O95630 |
| 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1 | HSD3B1 | - | C | Oxidation and isomerization in the biosynthesis of hormonal steroids | P14060 |
| 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 2 | HSD3B2 | - | C | Oxidation and isomerization in the biosynthesis of hormonal steroids | P26439 |
| Glycogen synthase kinase-3 beta | GSK3B | - | C | Protein tau phosphorylation | P49841 |
| Arf-GAP with Rho-GAP domain, ANK repeat and PH domain-containing protein 1 | ARAP1 | - | C | Reorganization of actin cytoskeleton | Q96P48 |
| DNA-directed RNA polymerase II subunit RPB1 | POLR2A | - | C | Transcription of DNA into RNA | P24928 |
| Exocyst complex component 5 | EXOC5 | - | C | Involved in exocytosis | O00471 |
| Alcohol dehydrogenase 4 | ADH4 | - | C | Oxidoreductase activity | A0A0D9SFB5 |
| Developmentally regulated GTP-binding protein 2 | DRG2 | - | C | Maintenance of cell structure and protein trafficking | P55039 |
| STAM-binding protein | STAMBP | - | C | Signal transduction for cell growth | O95630 |
| Cirhin | CIRH1A | - | C | Pre-ribosomal RNA transcription | L0R599 |
| F-actin-capping protein subunit beta | CAPZB | - | C | Cytoskeletal organization | P47756 |
| Coronin-2A | CORO2A | - | C | Actin filament binding | Q92828 |
| U6 snRNA-associated Sm-like protein LSm2 | LSM2 | - | C | Involved in RNA-binding proteins | Q9Y333 |
| Cytoplasmic dynein 1 heavy chain 1 | DYNC1H1 | 0.044 | 0.53 | Motility of vesicles through microtubules | Q14204 |
| Delta (24)-sterol reductase | DHCR24 | 0.037 | 0.55 | Protection against oxidative stress and apoptosis | Q15392 |
| Interferon regulatory factor 3 | IRF3 | 0.020 | 0.63 | Related to immunity | Q14653 |
| Myosin-10 | MYH14 | 0.036 | 0.68 | Cytoskeleton reorganization | P35580 |
| Lipopolysaccharide-responsive and beige-like anchor protein | LRBA | 0.026 | 0.70 | Vesicle trafficking of immune effector | P50851 |
| AP-1 complex subunit sigma-1A | AP1S1 | 0.002 | 0.71 | Related to clathrin | P61966 |
| DNA-directed RNA polymerase II subunit RPB2 | POLR2B | 0.008 | 0.71 | Component of RNA polymerase II | P30876 |
| Histone H2B tipo 1-L | H2BC13 | 0.048 | 0.73 | Histone related to nucleosome | Q99880 |
| Histone H2B type 1-M | H2BC14 | 0.048 | 0.73 | Histone related to nucleosome | Q99879 |
| Histone H2B type 1-N | H2BC15 | 0.048 | 0.73 | Histone related to nucleosome | Q99877 |
| Histone H2B type 2-F | H2BC18 | 0.048 | 0.73 | Histone related to nucleosome | Q5QNW6 |
| Histone H2B type 1-D | H2BC5 | 0.048 | 0.73 | Histone related to nucleosome | P58876 |
| Histone H2B type 1-H | H2BC9 | 0.048 | 0.73 | Histone related to nucleosome | Q93079 |
| D-dopachrome decarboxylase | DDT | 0.024 | 0.74 | Tautomerization of D-dopachrome | P30046 |
| D-dopachrome decarboxylase-like protein | DDTL | 0.024 | 0.74 | Lyase activity | A6NHG4 |
| Eukaryotic translation initiation factor 3 subunit D | EIF3D | 0.022 | 0.75 | Early steps of protein synthesis | O15371 |
| Sorting nexin-9 | SNX9 | 0.009 | 0.75 | Intracellular vesicle trafficking | O75116 |
| SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 5 | SMARCA5 | 0.049 | 0.78 | Helicase involved in nucleosome-remodeling activity | O60264 |
| Ribosome-binding protein 1 | RRBP1 | 0.017 | 0.78 | Mediates interaction between ribosome and endoplasmic reticulum membrane | Q9P2E9 |
| Coatomer subunit alpha | COPA | 0.026 | 0.79 | Formation of blebs in Golgi apparatus | P53621 |
| Dynactin subunit 1 | DCTN1 | 0.046 | 0.82 | Cofactor involved in microtubule motor cytoplasmic dynein | Q14203 |
| Eukaryotic translation initiation factor 2 subunit 3 | EIF2S3 | 0.045 | 0.86 | Early steps of protein synthesis | P41091 |
| Eukaryotic translation initiation factor 2 subunit 3B | EIF2S3B | 0.045 | 0.86 | Early steps of protein synthesis | Q2VIR3 |
| Beta-centractin | ACTR1B | 0.026 | 0.87 | Motility of vesicles through microtubules | P42025 |
| Leukocyte elastase inhibitor | SERPINB1 | 0.037 | 0.87 | Inflammatory caspase activation | P30740 |
| Thioredoxin reductase 1, cytoplasmic | TXNRD1 | 0.032 | 1.15 | Regulation of cellular redox and reductase activity on H2O2 | Q16881 |
| Calmodulin-1; Calmodulin-2; Calmodulin-3 | CALM1, CALM2, CALM3 | 0.009 | 1.29 | Involved in nitric oxide synthase regulator activity, adenylate cyclase activator activity and positive, negative regulation of ryanodine-sensitive calcium-release channel activity, type 3 metabotropic glutamate receptor binding and N-terminal myristoylation domain binding | P0DP23; P0DP24; P0DP25 |
| Mitochondrial glutamate carrier 1 | SLC25A22 | 0.002 | 1.30 | Mitochondrial carrier of glutamate | E9PJH7 |
| 60S ribosomal protein L11 | RPL11 | 0.033 | 1.57 | Component of the ribosome | P62913 |
| Cadherin-17 | CDH17 | 0.009 | 1.71 | Calcium-dependent cell adhesion protein involved in intestinal peptide transport | Q12864 |
| Tumor susceptibility gene 101 protein | TSG101 | 0.045 | 2.00 | Regulator of vesicular trafficking process | Q99816 |
| Serine/arginine repetitive matrix protein 1 | SRRM1 | 0.042 | 2.13 | Involved in numerous pre-mRNA processing events, part of pre- and post-splicing | Q8IYB3 |
| Neutral amino acid transporter B (0) | SLC1A5 | 0.005 | 2.22 | Sodium-dependent amino acids transporter including glutamine | Q15758 |
| U1 small nuclear ribonucleoprotein C | SNRPC | - | 3-NT | Component of the spliceosome | P09234 |
| CD2 antigen cytoplasmic tail-binding protein 2 | CD2BP2 | - | 3-NT | Involved in pre-mRNA splicing | O95400 |
| Carcinoembryonic antigen-related cell adhesion molecule 1 | CEACAM1 | - | 3-NT | Coinhibitory receptor in immune response | P13688 |
| La-related protein 4B | LARP4B | - | 3-NT | mRNA translation | Q92615 |
| Lipid droplet regulating VLDL assembly factor AUP1 | AUP1 | - | 3-NT | Translocation of misfolded proteins and degradation by the proteasome | Q9Y679 |
| Non-homologous end-joining factor 1 | NHEJ1 | - | 3-NT | DNA repair | Q9H9Q4 |
| DNA-directed RNA polymerase II subunit RPB3 | POLR2C | - | 3-NT | Transcription of DNA into RNA | P19387 |
| Protein Name | Gene Name | p-Value | Fold Change 1 | Biological Function | FASTA Accession Number |
|---|---|---|---|---|---|
| Translation initiation factor eIF-2B subunit alpha | EIF2B1 | - | 3-NT | Catalyzes the exchange of eukaryotic initiation factor 2-bound GDP for GTP | Q14232 |
| Eukaryotic translation initiation factor 2 subunit 2 | EIF2S2 | - | 3-NT | Involved in the early steps of protein synthesis along with GTP and tRNA | P20042 |
| La-related protein 1 | LARP1 | - | 3-NT | RNA-binding protein | Q6PKG0 |
| ATP synthase subunit delta, mitochondrial | ATP5F1D | - | 3-NT | Component of mitochondrial membrane ATP synthase | P30049 |
| MICOS complex subunit MIC13 | MICOS13 | - | 3-NT | Component of the MICOS complex, formation and maintenance of mitochondrial cristae | Q5XKP0 |
| Mitochondrial inner membrane protein OXA1L | OXA1L | - | 3-NT | Insertion of integral membrane proteins into the mitochondrial inner membrane | Q15070 |
| ATP-binding cassette sub-family B member 10, mitochondrial | ABCB10 | - | 3-NT | Exporting from the mitochondrial matrix to the cytosol in an ATP-dependent manner | Q9NRK6 |
| Ribosome-releasing factor 2, mitochondrial | GFM2 | - | 3-NT | Mitochondrial GTPase involved in mitochondrial protein biosynthesis | Q969S9 |
| Phosphatidylinositol-binding clathrin assembly protein | PICALM | - | 3-NT | Cytoplasmic adapter protein related to clathrin-mediated endocytosis | Q13492 |
| Plasma membrane calcium-transporting ATPase 4 | ATP2B4 | - | 3-NT | Calcium/calmodulin-regulated enzyme, catalyzes hydrolysis of ATP coupled with transport of calcium out of the cell | P23634 |
| Epidermal growth factor receptor | EGFR | - | 3-NT | Receptor tyrosine kinase binding | P00533 |
| MICOS complex subunit MIC19 | CHCHD3 | 1.41 × 10−5 | 0.07 | Component of the MICOS complex, maintenance of mitochondrial crista junctions | Q9NX63 |
| Histone H1.4 | H1-4 | 1.00 × 10−4 | 0.08 | Nucleosome assembly | P10412 |
| ATP synthase subunit alpha, mitochondrial | ATP5F1A | 0.010 | 0.17 | Component of mitochondrial membrane ATP synthase | P25705 |
| 60S ribosomal protein L13 | RPL13 | 0.011 | 0.22 | Component of the ribosome | P26373 |
| Dynamin-2 | DNM2 | 0.014 | 0.26 | Microtubule-associated protein, able to bind and hydrolyze GTP | P50570 |
| 60S ribosomal protein L23a | RPL23A | 1.80 × 10−3 | 0.26 | Component of the ribosome | P62750 |
| 60S ribosomal protein L12 | RPL12 | 1.86 × 10−6 | 0.27 | Component of the ribosome | P30050 |
| ATP synthase subunit beta, mitochondrial | ATP5F1B | 0.026 | 0.31 | Component of mitochondrial membrane ATP synthase | P06576 |
| 40S ribosomal protein S7 | RPS7 | 0.003 | 0.33 | Component of the ribosome | P62081 |
| DnaJ homolog subfamily C member 11 | DNAJC11 | 0.004 | 0.33 | Component of the MICOS complex, mitochondrial inner membrane organization | Q9NVH1 |
| Dynamin-like 120 kDa protein, mitochondrial | OPA1 | 4.21 × 10−5 | 0.34 | Regulates the equilibrium between mitochondrial fusion and mitochondrial fission | O60313 |
| Stomatin-like protein 2, mitochondrial | STOML2 | 2.00 × 10−4 | 0.38 | Mitochondrial protein involved in the activity of mitochondria | Q9UJZ1 |
| Adenylate kinase 4, mitochondrial | AK4 | 3.07 × 10−6 | 0.38 | Interconversion of nucleoside phosphates | P27144 |
| EF-hand domain-containing protein 1, mitochondrial | LETM1 | 1.60 × 10−4 | 0.40 | Mitochondrial proton/calcium antiporter, assembly of the supercomplexes of the respiratory chain | O95202 |
| Monocarboxylate transporter 1 | SLC16A1 | 9.00 × 10−4 | 0.41 | Proton-coupled monocarboxylate transporter | P53985 |
| ATP synthase subunit O, mitochondrial | ATP5PO | 2.39 × 10−5 | 0.42 | Component of mitochondrial membrane ATP synthase | P48047 |
| Eukaryotic initiation factor 4A-II | EIF4A2 | 9.13 × 10−5 | 0.43 | Required for mRNA binding to ribosome | Q14240 |
| Eukaryotic translation initiation factor 4E | EIF4E | 0.026 | 0.46 | Involved in protein synthesis and ribosome binding | P06730 |
| ATP synthase subunit d, mitochondrial | ATP5PD | 5.35 × 10−5 | 0.46 | Component of mitochondrial membrane ATP synthase | O75947 |
| MICOS complex subunit MIC60 | IMMT | 4.74 × 10−6 | 0.47 | Component of the MICOS complex, maintenance of mitochondrial cristae morphology | Q16891 |
| 60S ribosomal protein L11 | RPL11 | 0.035 | 0.49 | Component of the ribosome | P62913 |
| ATP synthase subunit gamma, mitochondrial | ATP5F1C | 3.1 × 10−4 | 0.50 | Component of mitochondrial membrane ATP synthase | P36542 |
| ATP synthase F (0) complex subunit B1, mitochondrial | ATP5PB | 1.63 × 10−5 | 0.50 | Component of mitochondrial membrane ATP synthase | P24539 |
| AFG3-like protein 2 | AFG3L2 | 4.1 × 10−3 | 0.50 | Involved in the regulation of OPA1 | Q9Y4W6 |
| Sorting and assembly machinery component 50 homolog | SAMM50 | 0.003 | 0.60 | Maintenance of the structure of mitochondrial cristae and the assembly of the mitochondrial respiratory chain complexes | Q9Y512 |
| Cytochrome c oxidase subunit 5B, mitochondrial | COX5B | 1.9 × 10−3 | 0.61 | Component of the cytochrome c oxidase | P10606 |
| Calcium/calmodulin-dependent protein kinase type II subunit beta | CAMK2B | 0.014 | 0.66 | Calcium/calmodulin-dependent protein kinase | Q13554 |
| Guanine nucleotide-binding protein G(s) subunit alpha isoforms XLas | GNAS | 0.008 | 0.67 | Transducer in pathways controlled by G protein-coupled receptors, activation of adenylate cyclase | Q5JWF2 |
| Heat shock protein HSP 90-beta | HSP90AB1 | 3.13 × 10−6 | 0.68 | Chaperone involved in cell cycle control and signal transduction | P08238 |
| Pyruvate kinase PKM | PKM | 4.1 × 10−4 | 0.7 | Glycolytic enzyme generating ATP and translation regulator of endoplasmic reticulum-associated ribosomes | P14618 |
| RNA-binding protein 4 | RBM4 | 0.007 | 0.73 | Splicing of pre-mRNA and translation regulation | Q9BWF3 |
| Calmodulin-1; Calmodulin-2; Calmodulin-3 | CALM1; CALM2; CALM3 | 0.022 | 0.73 | Nucleotide binding, 3 metabotropic glutamate receptor binding, N-terminal myristoylation domain binding, adenylate cyclase activator activity and nitric oxide synthase regulator activity | P0DP23; P0DP24; P0DP25 |
| Calcium-binding mitochondrial carrier protein Aralar2 | SLC25A13 | 4.1 × 10−4 | 0.74 | Mitochondrial and calcium-binding carrier, exchange cytoplasmic glutamate with mitochondrial aspartate | Q9UJS0 |
| Heat shock protein HSP 90-alpha | HSP90AA1 | 0.008 | 0.82 | Chaperone involved in cell cycle control and signal transduction | P07900 |
| Proteasome subunit alpha type 1 | PSMA1 | 0.009 | 1.18 | Component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P25786 |
| Proteasome subunit beta type-5 | PSMB5 | 0.002 | 1.19 | Component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P28074 |
| Protein disulfide-isomerase A4 | PDIA4 | 0.017 | 1.21 | Catalyzes the rearrangement of -S-S- bonds | P13667 |
| Aldo-keto reductase family 1 member A1 | AKR1A1 | 0.013 | 1.24 | Catalyzes the NADPH-dependent reduction of carbonyl-containing compounds, detoxifying enzyme | P14550 |
| Glutathione synthetase | GSS | 0.039 | 1.25 | Catalyzes the production of glutathione | P48637 |
| Glutathione reductase, mitochondrial | GSR | 0.007 | 1.25 | Maintains high levels of reduced glutathione in the cytosol | P00390 |
| Proteasome subunit beta type 4 | PSMB4 | 0.015 | 1.32 | Non-catalytic component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P28070 |
| Glutaredoxin-related protein 5, mitochondrial | GLRX5 | 0.032 | 1.33 | Involved in mitochondrial iron-sulfur cluster transfer | Q86SX6 |
| Serine hydroxymethyltransferase, mitochondrial | SHMT2 | 0.003 | 1.34 | Catalyzes the cleavage of serine to glycine | P34897 |
| Aflatoxin B1 aldehyde reductase member 2 | AKR7A2 | 0.002 | 1.35 | NADPH-dependent aldehyde reductase activity | O43488 |
| Proteasome subunit beta type 1 | PSMB1 | 0.002 | 1.40 | Non-catalytic component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P20618 |
| 3-hydroxyacyl-CoA dehydrogenase type-2 | HSD17B10 | 0.007 | 1.42 | Hydroxysteroid dehydrogenase activity in steroid hormones | Q99714 |
| Thioredoxin-like protein 1 | TXNL1 | 1.5 × 10−4 | 1.44 | Disulfide oxidoreductase activity | O43396 |
| Replication protein A 70 kDa DNA-binding subunit | RPA1 | 1.4 × 10−4 | 1.45 | DNA replication and response to DNA damage | P27694 |
| Sorbitol dehydrogenase | SORD | 1.3 × 10−3 | 1.46 | Catalyzes the reversible NAD+-dependent oxidation of sugar alcohols, a key enzyme in the polyol pathway | Q00796 |
| Proteasome subunit beta type 3 | PSMB3 | 0.008 | 1.55 | Non-catalytic component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P49720 |
| Proteasome subunit alpha type 6 | PSMA6 | 9.00 × 10−4 | 1.62 | Component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P60900 |
| Alpha-N-acetylgalactosaminidase | NAGA | 0.004 | 1.64 | Involved in the breakdown of glycolipids | P17050 |
| Alcohol dehydrogenase class-3 | ADH5 | 0.021 | 1.71 | Catalyzes the oxidation of S-(hydroxymethyl) glutathione | P11766 |
| Cathepsin B | CTSB | 0.016 | 1.72 | Catabolism of intracellular proteins | P07858 |
| Thioredoxin | TXN | 0.001 | 1.73 | Involved in antioxidant reactions | P10599 |
| Serine hydroxymethyltransferase, cytosolic | SHMT1 | 2.3 × 10−4 | 1.74 | Interconversion of serine and glycine | P34896 |
| Selenium-binding protein 1 | SELENBP1 | 0.006 | 1.75 | Catalyzes the oxidation of methanethiol | Q13228 |
| Lysosomal Pro-X carboxypeptidase | PRCP | 0.005 | 1.77 | Cleavage of amino acids | P42785 |
| Leukotriene A-4 hydrolase | LTA4H | 6.06 × 10−5 | 1.78 | Aminopeptidase activity | P09960 |
| Proteasome subunit beta type 2 | PSMB2 | 0.008 | 1.79 | Non-catalytic component of proteasome complex involved in the proteolytic degradation of most intracellular proteins | P49721 |
| Succinate dehydrogenase [ubiquinone] iron–sulfur subunit, mitochondrial | SDHB | 1.7 × 10−3 | 1.87 | Iron–sulfur protein subunit of succinate dehydrogenase, involved in complex II of the mitochondrial electron transport chain | P21912 |
| N-acetylated-alpha-linked acidic dipeptidase 2 | NAALAD2 | 0.022 | 1.87 | Dipeptidase activity | Q9Y3Q0 |
| Aspartyl aminopeptidase | DNPEP | 6.67 × 10−6 | 1.98 | Protein metabolism | Q9ULA0 |
| Replication protein A 14 kDa subunit | RPA3 | 0.003 | 2.02 | Controls DNA repair and DNA damage checkpoint activation | P35244 |
| Ubiquitin-40S ribosomal protein S27a | RPS27A | 0.002 | 2.02 | Postreplication repair | P62979 |
| Ubiquitin-60S ribosomal protein L40 | UBA52 | 0.002 | 2.02 | Postreplication repair | P62987 |
| Polyubiquitin-B | UBB | 0.002 | 2.02 | Postreplication repair | P0CG47 |
| Polyubiquitin-C | UBC | 0.002 | 2.02 | Postreplication repair | P0CG48 |
| Fatty aldehyde dehydrogenase | ALDH3A2 | 2.96 × 10−5 | 2.08 | Catalyzes the oxidation of aliphatic aldehydes to fatty acids | P51648 |
| Thioredoxin domain-containing protein 17 | TXNDC17 | 3.91 × 10−6 | 2.19 | Disulfide reductase with peroxidase activity | Q9BRA2 |
| Glycine dehydrogenase (decarboxylating), mitochondrial | GLDC | 2.17 × 10−5 | 2.23 | Catalyzes the degradation of glycine | P23378 |
| Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial | ALDH4A1 | 0.009 | 2.42 | Involved in the interconnexion between the urea and tricarboxylic acid cycles | P30038 |
| Catalase | CAT | 5.92 × 10−6 | 2.66 | Protection against oxidative stress | P04040 |
| Proliferating cell nuclear antigen | PCNA | 0.002 | 2.86 | DNA replication and DNA repair | P12004 |
| Tissue alpha-L-fucosidase | FUCA1 | 0.001 | 2.86 | Related to glycoside metabolic process | P04066 |
| Aldo-keto reductase family 1 member C3 | AKR1C3 | 2.83 × 10−5 | 3.21 | Oxidoreductase activity | P42330 |
| Dipeptidyl peptidase 2 | DPP7 | 1.24 × 10−6 | 3.40 | Degradation of tripeptides | Q9UHL4 |
| DNA ligase 3 | LIG3 | - | 3-NT+EA | Correct defective DNA strand-break repair | P49916 |
| Alcohol dehydrogenase 4 | ADH4 | - | 3-NT+EA | Oxidoreductase activity | P08319 |
| DNA-directed RNA polymerase II subunit RPB1 | POLR2A | - | 3-NT+EA | Transcription of DNA into RNA | P24928 |
| Glutathione S-transferase Mu 3 | GSTM3 | - | 3-NT+EA | Detoxification of endogenous compounds | P21266 |
| Alcohol dehydrogenase 4 | ADH4 | - | 3-NT+EA | Oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | A0A0D9SFB5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Velasco, S.; Delgado, J.; Peña, F.J.; Estévez, M. Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study. Antioxidants 2022, 11, 2485. https://doi.org/10.3390/antiox11122485
Díaz-Velasco S, Delgado J, Peña FJ, Estévez M. Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study. Antioxidants. 2022; 11(12):2485. https://doi.org/10.3390/antiox11122485
Chicago/Turabian StyleDíaz-Velasco, Silvia, Josué Delgado, Fernando J. Peña, and Mario Estévez. 2022. "Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study" Antioxidants 11, no. 12: 2485. https://doi.org/10.3390/antiox11122485