Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation—Potential Risk for Impaired Wound Healing in Smokers
Abstract
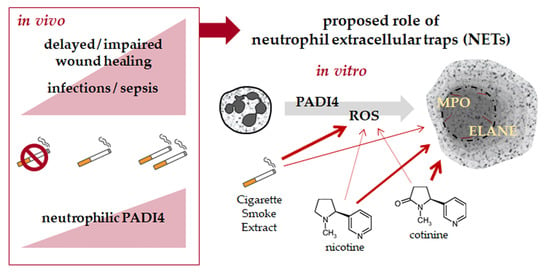
:1. Introduction
2. Materials and Methods
2.1. Patient Data and Human Material
2.2. Isolation of Neutrophils
2.3. PADI4 Gene Expression
2.4. Stimulation of Neutrophils
2.5. SYTOXTM Green Assay
2.6. Immuno-Fluorescent Staining
2.7. DCFH-DA Assay
2.8. Enzyme Activity Assays
2.8.1. Superoxide Dismutase (SOD) Activity Assay
2.8.2. Catalase Activity Assay
2.8.3. Glutathione Peroxidase (GPx) Activity Assay
2.8.4. Glutathione Reductase (GR) Activity Assay
2.8.5. Peptidyl-Arginine-Deiminase 4 (PADI4) Activity Assay
2.8.6. Myeloperoxidase (MPO) Activity Assay
2.8.7. Neutrophilic Elastase (ELANE) Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Smoking Increases the Complication Rates in Orthopedic and Trauma Patients
3.2. Patient Characteristics
3.3. Neutrophilic PADI4 Expression Is Increased in Heavy and Active Smokers
3.4. CSE, Nicotine, and Cotinine Moderately Induce NET Formation
3.5. CSE Induces Oxidative Stress in Neutrophils and Interferes with the Cells Antioxidative Defense
3.6. CSE, Nicotine and Cotinine Affect the Activity of Enzymes Involved in NET Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.E.; Carstensen, S.E.; Moore, S.; Dacus, A.R. Smoking Increases Postoperative Complications after Distal Radius Fracture Fixation: A Review of 417 Patients from a Level 1 Trauma Center. Hand 2018, 15, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schroter, S.; Relja, B.; Nussler, A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolaro, J.A.; Schenker, M.L.; Yannascoli, S.; Baldwin, K.; Mehta, S.; Ahn, J. Cigarette smoking increases complications following fracture: A systematic review. J. Bone Jt. Surg. Am. 2014, 96, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Mosely, L.H.; Finseth, F. Cigarette smoking: Impairment of digital blood flow and wound healing in the hand. Hand 1977, 9, 97–101. [Google Scholar] [CrossRef]
- Silverstein, P. Smoking and wound healing. Am. J. Med. 1992, 93, 22S–24S. [Google Scholar] [CrossRef]
- Whiteford, L. Nicotine, CO and HCN: The detrimental effects of smoking on wound healing. Br. J. Community Nurs. 2003, 8, S22–S26. [Google Scholar] [CrossRef]
- Njeim, R.; Azar, W.S.; Fares, A.H.; Azar, S.T.; Kfoury Kassouf, H.; Eid, A.A. NETosis contributes to the pathogenesis of diabetes and its complications. J. Mol. Endocrinol. 2020, 65, R65–R76. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- White, P.C.; Hirschfeld, J.; Milward, M.R.; Cooper, P.R.; Wright, H.J.; Matthews, J.B.; Chapple, I.L.C. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J. Periodontal Res. 2018, 53, 525–535. [Google Scholar] [CrossRef]
- Perez-Olivares, L.; Soehnlein, O. Contemporary Lifestyle and Neutrophil Extracellular Traps: An Emerging Link in Atherosclerosis Disease. Cells 2021, 10, 1985. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, S.L.; Tang, Q.Y.; Zhou, X.; Zhang, J.Q.; He, Z.Y.; Bai, J.; Li, M.H.; Deng, J.M.; Liang, Y.; et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, R.; Herbert, G.; Atkinson, C.; England, C.; Northstone, K.; Baos, S.; Brush, T.; Chong, A.; Ness, A.; Harris, J.; et al. Pre-admission interventions (prehabilitation) to improve outcome after major elective surgery: A systematic review and meta-analysis. BMJ Open 2021, 11, e050806. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Aubin, H.J. Do placebo response rates from cessation trials inform on strength of addictions? Int. J. Environ. Res. Public Health 2012, 9, 192–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mersha, A.G.; Eftekhari, P.; Bovill, M.; Tollosa, D.N.; Gould, G.S. Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: A systematic review and meta-analysis. Arch. Public Health 2021, 79, 26. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, C.; Venturelli, S.; Konrad, F.; Nussler, A.K.; Ehnert, S. Bio-impedance measurement allows displaying the early stages of neutrophil extracellular traps. Excli J. 2020, 19, 1481–1495. [Google Scholar] [PubMed]
- Vigano, M.; Perucca Orfei, C.; de Girolamo, L.; Pearson, J.R.; Ragni, E.; De Luca, P.; Colombini, A. Housekeeping Gene Stability in Human Mesenchymal Stem and Tendon Cells Exposed to Tenogenic Factors. Tissue Eng. Part C Methods 2018, 24, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Han, W.; Giraldo, C.; De Li, Y.; Block, E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; Fentz, A.K.; Schreiner, A.; Birk, J.; Wilbrand, B.; Ziegler, P.; Reumann, M.K.; Wang, H.; Falldorf, K.; Nussler, A.K. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of •O2− and H2O2. Sci. Rep. 2017, 7, 14544. [Google Scholar] [CrossRef] [Green Version]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Yang, W.; Burkhardt, B.; Fischer, L.; Beirow, M.; Bork, N.; Wonne, E.C.; Wagner, C.; Husen, B.; Zeilinger, K.; Liu, L.; et al. Age-dependent changes of the antioxidant system in rat livers are accompanied by altered MAPK activation and a decline in motor signaling. Excli J. 2015, 14, 1273–1290. [Google Scholar] [PubMed]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Sabulski, M.J.; Fura, J.M.; Pires, M.M. Fluorescence-based monitoring of PAD4 activity via a pro-fluorescence substrate analog. J. Vis. Exp. 2014, 5, e52114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stemmelin, G.R.; Doti, C.A.; Shanley, C.M.; Ceresetto, J.M.; Rabinovich, O.M.; Vicente Reparaz, M.A.; Vukovic, M.G.; Bullorsky, E.O. Smoking as a Cause for Mild Chronic Neutrophilia. Blood 2004, 104, 3796. [Google Scholar] [CrossRef]
- Chaiton, M.; Diemert, L.; Cohen, J.E.; Bondy, S.J.; Selby, P.; Philipneri, A.; Schwartz, R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 2016, 6, e011045. [Google Scholar] [CrossRef] [Green Version]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018, 5, CD000146. [Google Scholar] [CrossRef]
- Samara, K.D.; Trachalaki, A.; Tsitoura, E.; Koutsopoulos, A.V.; Lagoudaki, E.D.; Lasithiotaki, I.; Margaritopoulos, G.; Pantelidis, P.; Bibaki, E.; Siafakas, N.M.; et al. Upregulation of citrullination pathway: From Autoimmune to Idiopathic Lung Fibrosis. Respir. Res. 2017, 18, 218. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, L.C.; Konig, M.F.; Gelber, A.C.; Bingham, C.O., 3rd; Darrah, E. Smoking is not linked to the development of anti-peptidylarginine deiminase 4 autoantibodies in rheumatoid arthritis. Arthritis Res. 2018, 20, 59. [Google Scholar] [CrossRef] [Green Version]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Munzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009, 29–60. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, A.; Thompson, P.R.; Segal, B.H.; Urban, C.F. Nicotine induces neutrophil extracellular traps. J. Leukoc. Biol. 2016, 100, 1105–1112. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnen, M.; Moller, S.; Brozek, A.; Klinger, M.; Laskay, T. Extracellular Acidification Inhibits the ROS-Dependent Formation of Neutrophil Extracellular Traps. Front. Immunol. 2017, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir-Kumral, Z.N.; Ozbeyli, D.; Ozdemir, A.F.; Karaaslan, B.M.; Kaytaz, K.; Kara, M.F.; Tok, O.E.; Ercan, F.; Yegen, B.C. Protective Effect of Nicotine on Sepsis-Induced Oxidative Multiorgan Damage: Role of Neutrophils. Nicotine Tob. Res. 2017, 19, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.F.; Tian, F.; Lu, Z.F.; Yang, G.D.; Fu, H.Y.; Wang, J.; Yan, X.L.; Zhao, Y.C.; Wang, Y.J.; Jiang, T. Protective effect of nicotine on lipopolysaccharide-induced acute lung injury in mice. Respiration 2011, 81, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Fant, R.V.; Buchhalter, A.R.; Gitchell, J.G.; Henningfield, J.E. Nicotine delivery systems. Expert Opin. Drug Deliv. 2005, 2, 563–577. [Google Scholar] [CrossRef]
- Mayer, B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch. Toxicol. 2014, 88, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.A.; Danna-Lopes, D.; Sarfati, I.; Rao, S.K.; Cohen, J.R. Nicotine-stimulated elastase activity release by neutrophils in patients with abdominal aortic aneurysms. Ann. Vasc. Surg. 1998, 12, 41–45. [Google Scholar] [CrossRef]







| Non-Smokers | Moderate Smokers | Heavy Smokers | All | |
|---|---|---|---|---|
| Number of patients [N] | 1199 | 684 | 435 | 2318 |
| Male [N] | 561 (46.8%) | 443 (64.8%) | 304 (69.9%) | 1308 (56.4%) |
| Female [N] | 638 (53.2%) | 241 (35.2%) | 131 (30.1%) | 1010 (43.6%) |
| Age [years] | 59.0 ± 18.6 (18–96) | 53.0 ± 17.8 (18–97) | 58.0 ± 11.9 (24–91) | 57.0 ± 17.5 (18–97) |
| Body mass index [kg/m2] | 27.3 ± 5.3 (16.0–52.3) | 27.6 ± 5.4 (15.6–60.8) | 28.2 ± 5.7 (14.0–48.3) | 27.6 ± 5.4 (14.0–60.8) |
| Diabetes mellitus [N] | 149 (12.4%) | 65 (9.5%) | 88 (20.2%) | 302 (13.0%) |
| Complications with: | ||||
| Wound healing [N] | 145 (12.1%) | 110 (16.1%) | 100 (23.0%) | 355 (15.3%) |
| Infections/Sepsis [N] | 106 (8.8%) | 70 (10.2%) | 90 (20.7%) | 266 (11.5%) |
| Bone healing [N] | 87 (7.3%) | 55 (8.0%) | 69 (15.9%) | 211 (9.1%) |
| Implant [N] | 64 (5.3%) | 24 (3.5%) | 47 (10.8%) | 135 (5.8%) |
| Others [N] | 170 (14.2%) | 117 (17.1%) | 92 (21.1%) | 379 (16.4%) |
| Target | GenID | Primer | Efficiency | Amplicon | TA |
|---|---|---|---|---|---|
| EF1α | NM_001402.5 | For-CCCCGACACAGTAGCATTTG | 1.90 | 98 bp | 56 °C |
| Rev-TGACTTTCCATCCCTTGAACC | |||||
| RPL13a | NM_012423.3 | For-AAGTACCAGGCAGTGACAG | 2.24 | 100 bp | 56 °C |
| Rev-CCTGTTTCCGTAGCCTCATG | |||||
| PADI4 | NM_012387.2 | For-AGAGGTGACCCTGACGATGA | 2.09 | 310 bp | 56 °C |
| Rev-CAGGTCTTCGCTGTCAAGCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspera-Werz, R.H.; Mück, J.; Linnemann, C.; Herbst, M.; Ihle, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation—Potential Risk for Impaired Wound Healing in Smokers. Antioxidants 2022, 11, 2424. https://doi.org/10.3390/antiox11122424
Aspera-Werz RH, Mück J, Linnemann C, Herbst M, Ihle C, Histing T, Nussler AK, Ehnert S. Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation—Potential Risk for Impaired Wound Healing in Smokers. Antioxidants. 2022; 11(12):2424. https://doi.org/10.3390/antiox11122424
Chicago/Turabian StyleAspera-Werz, Romina H., Jonas Mück, Caren Linnemann, Moritz Herbst, Christoph Ihle, Tina Histing, Andreas K. Nussler, and Sabrina Ehnert. 2022. "Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation—Potential Risk for Impaired Wound Healing in Smokers" Antioxidants 11, no. 12: 2424. https://doi.org/10.3390/antiox11122424