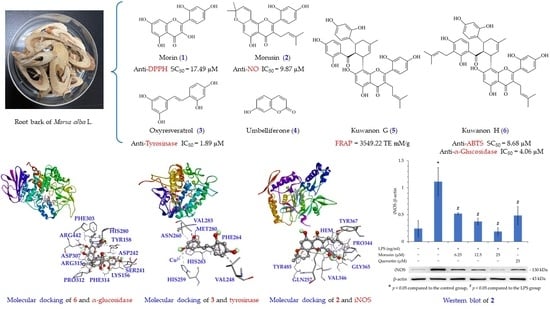
Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Preparation of Morus alba L. Extract
2.3. Preparation of Active Components
2.4. Reverse-Phase HPLC
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. DPPH Radical Scavenging Activity
2.8. ABTS Radical Scavenging Activity
2.9. Superoxide Radical Scavenging Activity
2.10. Ferric Reducing Antioxidant Power (FRAP)
2.11. α-Glucosidase Inhibitory Activity Assay
2.12. Tyrosinase Inhibitory Activity Assay
2.13. Cell Culture
2.14. Nitric Oxide Inhibitory Assay
2.15. MTT Assay
2.16. Western Blot Analysis
2.17. Molecular Modeling Docking Study
2.18. Statistical Analysis
3. Results
3.1. Determination of TPC, TFC and Yields in Each Solvent Extract
3.2. DPPH Free Radical Scavenging Activity
3.3. ABTS Free Radical Scavenging Activity
3.4. Superoxide Radical Scavenging Activity
3.5. Ferric Reducing Antioxidant Power
3.6. Anti-α-Glucosidase Activity Assay
3.7. Antityrosinase Activity Assay
3.8. Anti-Inflammatory Activity of Solvents
3.9. Cell Viability of Extracts
3.10. Quantitation of Active Components in Solvent Extracts
3.11. Antioxidant Activities of Isolated Components
3.12. Anti-α-Glucosidase Activities of Isolated Components
3.13. Antityrosinase Activities of Isolated Components
3.14. Anti-Inflammatory Activities of Isolated Components
3.15. Cell Viability of Isolated Components
3.16. Western Blot Analysis of Isolated Components
3.17. Molecular Modeling Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witschi, H. Enhanced tumour development by butylated hydroxytoluene (BHT) in the liver, lung and gastro-intestinal tract. Food Chem. Toxicol. 1986, 24, 1127–1130. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology 2001, 169, 1–15. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Wagle, A.; Min, B.S.; Jung, H.A.; Choi, J.S. Antioxidant and anti-browning property of 2-arylbenzofuran derivatives from Morus alba Linn root bark. Food Chem. 2020, 309, 125739. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging targets in type 2 diabetes and diabetic complications. Adv. Sci. 2021, 8, 2100275. [Google Scholar] [CrossRef]
- Lebovitz, H.E. alpha-Glucosidase inhibitors. Endocrinol. Metabol. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Phcog. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaoxue, X.; Yanjun, D.; Zhongyi, X.; Leihong, X. Implications of oxidative stress in the pathogenesis and treatment of hyperpigmentation disorders. Oxidative Med. Cell. Longev. 2022, 2022, 7881717. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juanjuan, C.; Yang, L.; Zhao, Z.; Jie, Q. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [Green Version]
- You, S.; Jang, M.; Kim, G.H. Mori Cortex Radicis Attenuates High Fat Diet-Induced Cognitive Impairment via an IRS/Akt Signaling Pathway. Nutrients 2020, 12, 1851. [Google Scholar] [CrossRef]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Nam, S.Y.; Yi, H.K.; Lee, J.C.; Kim, J.C.; Song, C.H.; Park, J.W.; Lee, D.Y.; Kim, J.S.; Hwang, P.H. Cortex mori extract induces cancer cell apoptosis through inhibition of microtubule assembly. Arch. Pharm. Res. 2002, 25, 191–196. [Google Scholar] [CrossRef]
- Hyun, J.; Im, J.; Kim, S.W.; Kim, H.Y.; Seo, I.; Bhang, S.H. Morus alba root extract induces the anagen phase in the human hair follicle dermal papilla cells. Pharmaceutics 2021, 13, 1155. [Google Scholar] [CrossRef]
- Park, S.; Moon, B.R.; Kim, J.E.; Kim, H.J.; Zhang, T. Aqueous extracts of Morus alba root bark and Cornus officinalis fruit protect against osteoarthritis symptoms in testosterone-deficient and osteoarthritis-induced rats. Pharmaceutics 2020, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, T.M.; Šiler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Antioxidant activity and total phenolic content in some cereals and legumes. Int. J. Food Prop. 2011, 14, 175–184. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Singh, A.P. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Sivasothy, Y.; Loo, K.Y.; Leong, K.H.; Litaudon, M.; Awang, K. A potent alpha-glucosidase inhibitor from Myristica cinnamomea King. Phytochemistry 2016, 112, 265–269. [Google Scholar] [CrossRef]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, PL241–PL246. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, S.S.; Chen, S.C.; Ho, F.M.; Lin, W.W. Oregonin inhibits lipopolysaccharide-induced iNOS gene transcription and upregulates HO-1 expression in macrophages and microglia. Br. J. Pharmacol. 2005, 146, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- BIOVIA; Dassault Systèmes. Discovery Studio Client 2021, v.21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Tagami, T.; Yamashita, K.; Okuyama, M.; Mori, H.; Yao, M.; Kimura, A. Molecular basis for the recognition of long-chain substrates by plant α-glucosidase. J. Biol. Chem. 2013, 288, 19296–19303. [Google Scholar] [CrossRef] [Green Version]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, R.J.; Garcin, E.D.; Panda, K.; Andersson, G.; Åberg, A.; Wallace, A.V.; Morris, G.M.; Olson, A.J.; Stuehr, D.J.; Tainer, J.A. Conformational changes in nitric oxide synthases induced by chlorzoxazone and nitroindazoles: Crystallographic and computational analyses of inhibitor potency. Biochemistry 2002, 41, 13915–13925. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.M.; Mittal, A.; Sharma, M.; Bharatam, P.V. Design of Benzene-1, 2-diamines as selective inducible nitric oxide synthase inhibitors: A combined de novo design and docking analysis. J. Mol. Model. 2008, 14, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Zaheer, J.; Najam-Us-Saqib, Q.; Qamar, M.; Akram, M. In vitro (anti-alpha-glucosidase) activity and in vivo anti-diabetic activity of Androsace foliosa (common rock jasmine) in alloxan-induced diabetic BALB/c mice. Eur. J. Inflamm. 2019, 17, 2058739219857429. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. A determination of potential α-glucosidase inhibitors from azuki beans (Vigna angularis). Int. J. Mol. Sci. 2011, 12, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Negroiu, G.; Petrescu, A.J.; Garman, E.F.; Platt, F.M.; Wormald, M.R.; Dwek, R.A.; Petrescu, S.M. Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J. Biol. Chem. 2000, 275, 8169–8175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, M.; Oshima, T.; Koshio, K.; Itsuzaki, Y.; Anzai, J. Tyrosinase inhibitor from black rice bran. J. Agric. Food Chem. 2003, 51, 6953–6956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ha, S.-H.; Abekura, F.; Lim, H.; Magae, J.; Ha, K.-T.; Chung, T.-W.; Chang, Y.-C.; Lee, Y.-C.; Chung, E. 4-O-Carboxymethylascochlorin inhibits expression levels of on inflammation-related cytokines and matrix metalloproteinase-9 Through NF–κB/MAPK/TLR4 signaling pathway in LPS-activated RAW264. 7 cells. Front. Pharmacol. 2019, 10, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cha, H.-J.; Lee, H.; Kim, G.-Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]












| Extracting Solvent | Relative Polarity | TPC (mg/g) a (GAE) | TFC (mg/g) b (QE) | Yield (%) c |
|---|---|---|---|---|
| n-Hexane | 0.009 | 0.00 | 99.58 ± 2.65 *** | 1.5 ± 0.49 |
| Dichloromethane | 0.269 | 13.63 ± 0.33 *** | 114.36 ± 4.94 *** | 1.6 ± 0.61 |
| Ethyl acetate | 0.288 | 68.65 ± 6.12 ** | 156.20 ± 1.75 *** | 1.9 ± 0.35 |
| Acetone | 0.355 | 94.61 ± 2.36 *** | 167.22 ± 1.26 *** | 2.0 ± 0.37 |
| Ethanol | 0.654 | 45.34 ± 0.77 *** | 117.77 ± 2.01 *** | 3.6 ± 0.84 |
| Methanol | 0.762 | 60.76 ± 1.41 *** | 54.12 ± 1.37 *** | 5.3 ± 1.19 |
| Water | 1.000 | 5.17 ± 1.48 * | 20.73 ± 1.63 * | 23.9 ± 5.50 |
| Extracting Solvent | SC50 (μg/mL) a | TE (mM/g) c | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| n-Hexane | >400 | >400 | >400 | 50.83 ± 0.91 *** |
| Dichloromethane | >400 | >400 | >400 | 98.39 ± 0.69 *** |
| Ethyl acetate | >400 | 159.16 ± 10.74 ** | 388.74 ± 10.26 ** | 197.31 ± 2.11 *** |
| Acetone | 242.33 ± 15.78 ** | 129.28 ± 10.53 ** | 124.80 ± 22.09 ** | 213.67 ± 2.06 *** |
| Ethanol | 331.81 ± 22.55 ** | 118.25 ± 3.29 ** | >400 | 181.83 ± 3.24 *** |
| Methanol | 308.10 ± 8.24 * | 65.60 ± 6.03 ** | 299.13 ± 26.45 * | 200.51 ± 2.53 *** |
| Water | >400 | 257.38 ± 32.53 * | >400 | 52.32 ± 1.91 *** |
| BHT b | 19.75 ± 1.79 * | 25.87 ± 5.83 * | N.A. d | 4385.56 ± 45.54 *** |
| Compound | IC50 (μg/mL) a | |
|---|---|---|
| α-Glucosidase | Tyrosinase | |
| n-Hexane | 22.85 ± 4.51 * | >400 |
| Dichloromethane | 12.04 ± 6.67 ** | >400 |
| Ethyl acetate | 5.80 ± 2.29 ** | 11.27 ± 2.75 ** |
| Acetone | 3.87 ± 1.95 *** | 7.95 ± 1.54 ** |
| Ethanol | 11.48 ± 1.81 * | 19.86 ± 3.35 ** |
| Methanol | 12.48 ± 0.93 ** | 13.41 ± 0.77 *** |
| Water | 17.94 ± 6.37 ** | >400 |
| Acarbose b | 355.48 ± 28.39 * | - |
| Arbutin b | - | 53.51 ± 8.87 * |
| Compound | NO Inhibition IC50 (μg/mL) a |
|---|---|
| n-Hexane | 53.22 ± 17.28 * |
| Dichloromethane | 15.33 ± 2.63 * |
| Ethyl acetate | 10.81 ± 1.41 * |
| Acetone | 12.00 ± 1.32 * |
| Ethanol | 14.30 ± 2.38 * |
| Methanol | 17.19 ± 2.04 * |
| Water | 121.08 ± 7.85 * |
| Quercetin b | 3.98 ± 0.28 * |
| Extracting Solvent | Morin (mg/g) | Morusin (mg/g) | Oxyresveratrol (mg/g) | Umbelliferone (mg/g) | Kuwanon G (mg/g) | Kuwanon H (mg/g) | Total Amount (mg/g) |
|---|---|---|---|---|---|---|---|
| n-Hexane | 2.23 ± 0.18 | 3.92 ± 0.25 | 0.64 ± 0.08 | 2.35 ± 0.16 | 11.27 ± 0.92 | 2.46 ± 0.18 | 22.87 ± 1.77 |
| Dichloromethane | 0.92 ± 0.10 | 9.96 ± 0.98 | 1.22 ± 0.13 | 2.93 ± 0.18 | 8.24 ± 0.88 | 5.43 ± 0.58 | 28.70 ± 4.31 |
| Ethyl acetate | 2.56 ± 0.42 | 7.81 ± 0.62 | 2.99 ± 0.19 | 3.29 ± 0.12 | 12.84 ± 1.02 | 8.32 ± 0.79 | 37.81 ± 3.16 |
| Acetone | 3.24 ± 0.26 | 8.64 ± 0.92 | 3.33 ± 0.22 | 2.23 ± 0.12 | 18.54 ± 1.82 | 21.24 ± 1.94 | 57.22 ± 5.28 |
| Ethanol | 3.51 ± 0.27 | 5.82 ± 0.47 | 2.65 ± 0.21 | 1.43 ± 0.07 | 19.04 ± 1.76 | 26.74 ± 2.24 | 59.19 ± 5.02 |
| Methanol | 1.60 ± 0.09 | 4.14 ± 0.36 | 3.17 ± 0.28 | 2.73 ± 0.13 | 22.64 ± 2.02 | 29.34 ± 2.83 | 63.62 ± 5.71 |
| Water | 2.87 ± 0.24 | 4.07 ± 0.48 | 2.41 ± 0.22 | 1.22 ± 0.18 | 18.38 ± 1.24 | N.D. | 28.95 ± 2.36 |
| Compound | SC50 (μM) a | TE (mM/g) c | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| Morin (1) | 17.49 ± 3.43 * | 12.67 ± 5.98 *** | 54.50 ± 18.96 * | 2963.28 ± 63.65 *** |
| Morusin (2) | >200 | 44.19 ± 8.07 *** | >200 | 583.43 ± 25.79 *** |
| Oxyresveratrol (3) | 44.42 ± 12.51 * | 9.50 ± 2.08 *** | >200 | 2402.22 ± 32.91 *** |
| Umbelliferone (4) | >200 | >200 | >200 | 145.23 ± 35.46 ** |
| Kuwanon G (5) | >200 | 9.28 ± 1.02 ** | 188.24 ± 19.07 * | 3549.22 ± 160.65 * |
| Kuwanon H (6) | >200 | 8.68 ± 0.93 ** | >200 | 1750.96 ± 36.30 ** |
| BHT b | 89.84 ± 7.03 ** | 115.86 ± 25.14 * | N.A. d | 4385.56 ± 78.88 *** |
| Compound | IC50 (μM) a | |
|---|---|---|
| α-Glucosidase | Tyrosinase | |
| Morin (1) | 90.15 ± 10.17 * | 199.41 ± 84.44 ** |
| Morusin (2) | 15.66 ± 2.15 ** | >400 |
| Oxyresveratrol (3) | 8.35 ± 0.46 * | 1.89 ± 0.59 ** |
| Umbelliferone (4) | 492.46 ± 12.82 * | >400 |
| Kuwanon G (5) | 7.00 ± 0.31 * | 100.40 ± 9.62 * |
| Kuwanon H (6) | 4.06 ± 0.12 * | 218.00 ± 40.96 * |
| Acarbose b | 540.38 ± 47.42 * | - |
| Arbutin b | - | 206.08 ± 36.50 * |
| Compound | NO Inhibition IC50 (μM) a |
|---|---|
| Morin (1) | 43.26 ± 4.61 * |
| Morusin (2) | 9.87 ± 0.59 * |
| Oxyresveratrol (3) | 25.36 ± 3.47 * |
| Umbelliferone (4) | 36.76 ± 2.26 * |
| Kuwanon G (5) | 17.80 ± 0.50 * |
| Kuwanon H (6) | 40.48 ± 4.38 * |
| Quercetin b | 13.20 ± 2.00 * |
| Compound | Affinity (kcal/mol) |
|---|---|
| Morin (1) | −7.6 |
| Morusin (2) | −8.0 |
| Oxyresveratrol (3) | −8.2 |
| Umbelliferone (4) | −5.9 |
| Kuwanon G (5) | −8.5 |
| Kuwanon H (6) | −9.9 |
| Acarbose a | −5.6 |
| Compound | Affinity (kcal/mol) |
|---|---|
| Morin (1) | −6.1 |
| Morusin (2) | −5.5 |
| Oxyresveratrol (3) | −7.3 |
| Umbelliferone (4) | −5.3 |
| Kuwanon G (5) | −6.4 |
| Kuwanon H (6) | −5.9 |
| Arbutin a | −6.1 |
| Compound | Affinity (kcal/mol) |
|---|---|
| Morin (1) | −7.0 |
| Morusin (2) | −9.7 |
| Oxyresveratrol (3) | −7.5 |
| Umbelliferone (4) | −6.9 |
| Kuwanon G (5) | −8.1 |
| Kuwanon H (6) | −7.3 |
| Quercetin a | −7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, J.-H.; Yang, C.-S.; Chen, J.-J. Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba. Antioxidants 2022, 11, 2222. https://doi.org/10.3390/antiox11112222
Hsu J-H, Yang C-S, Chen J-J. Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba. Antioxidants. 2022; 11(11):2222. https://doi.org/10.3390/antiox11112222
Chicago/Turabian StyleHsu, Jui-Hung, Chang-Syun Yang, and Jih-Jung Chen. 2022. "Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba" Antioxidants 11, no. 11: 2222. https://doi.org/10.3390/antiox11112222