Targeted UHPLC-MS Analysis Reveals Disparate Polyphenol Composition and Concentration in Muscadine Grape Supplements with Proportional Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Commercially Processed MGSs
2.3. Extraction of Phenolic Compounds
2.4. Total Phenolic Content (TPC)
2.5. Quantification of Antioxidant Capacity
2.6. UHPLC-MS Analysis
2.7. Quantification of Antioxidant Markers and Enzymes
2.8. Data and Statistical Analysis
3. Results
3.1. Total Phenolic Content (TPC) and Antioxidant Capacity of MGSs
3.2. UHPLC-MS of Commercially Processed MGSs
3.3. Polyphenol Content in Commercial MGSs
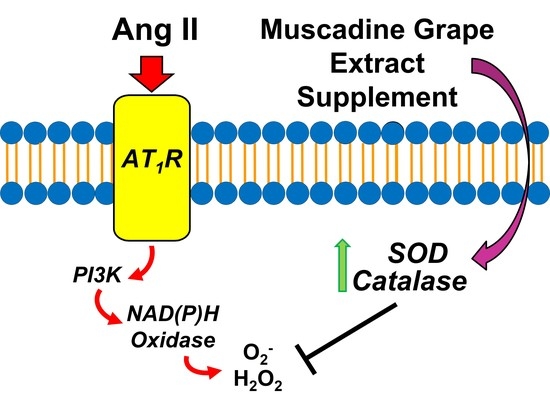
3.4. Effect of Commercially Prepared MGSs on Oxidative Stress
3.5. Effect of Commercially Prepared MGSs on Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bukhari, S.N.A. Dietary Polyphenols as Therapeutic Intervention for Alzheimer’s Disease: A Mechanistic Insight. Antioxidants 2022, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Jaiswal, S.; Ravindra, P.V. Modulation of gut microbiota by bioactive compounds for prevention and management of type 2 diabetes. Biomed. Pharmacother. 2022, 152, 113148. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, R.T.; Xiong, J.; Lila, M.A. Comparison of berry juice concentrates and pomaces and alternative plant proteins to produce spray dried protein-polyphenol food ingredients. Food Funct. 2019, 10, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) As determined by HPLC-DAD-ESI-MS(n). J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, B.; Eaves, D.H.; Shikany, J.M.; Pace, R.D. Total phenolics and antioxidant capacity of indigenous vegetables in the southeast United States: Alabama Collaboration for Cardiovascular Equality Project. Int. J. Food Sci. Nutr. 2009, 60, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Denmeade, S.R.; Carducci, M.A. Challenges of conducting clinical trials of natural products to combat cancer. Clin. Adv. Hematol. Oncol. 2016, 14, 447–455. [Google Scholar] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Luo, J.; Huang, Y.; Guo, W.; Zhang, Y.; Guan, H.; Xu, C.; Lu, J. Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. Int. J. Mol. Sci. 2017, 18, 631. [Google Scholar] [CrossRef] [Green Version]
- You, Q.; Chen, F.; Sharp, J.L.; Wang, X.; You, Y.; Zhang, C. High-performance liquid chromatography-mass spectrometry and evaporative light-scattering detector to compare phenolic profiles of muscadine grapes. J. Chromatogr. A 2012, 1240, 96–103. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Chen, F.; Wang, X.; Sharp, J.L.; You, Y. Analysis of phenolic composition of Noble muscadine (Vitis rotundifolia) by HPLC-MS and the relationship to its antioxidant capacity. J. Food Sci. 2012, 77, C1115–C1123. [Google Scholar] [CrossRef] [PubMed]
- Villani, T.S.; Reichert, W.; Ferruzzi, M.G.; Pasinetti, G.M.; Simon, J.E.; Wu, Q. Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration. Food Chem. 2015, 170, 271–280. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Bitting, R.L.; Tooze, J.A.; Isom, S.; Petty, W.J.; Grant, S.C.; Desnoyers, R.J.; Thomas, A.; Thomas, C.Y.; Alistar, A.T.; Golden, S.L.; et al. Phase I Study of Muscadine Grape Extract for Patients With Advanced Cancer. Am. J. Clin. Oncol. 2021, 44, 239–246. [Google Scholar] [CrossRef]
- Singleton, V. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.K.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Oxidative Stress as A Mechanism for Functional Alterations in Cardiac Hypertrophy and Heart Failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Tallant, E.A.; Ferrario, C.M.; Gallagher, P.E. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1560–H1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collard, M.; Gallagher, P.E.; Tallant, E.A. A Polyphenol-Rich Extract From Muscadine Grapes Inhibits Triple-Negative Breast Tumor Growth. Integr. Cancer Ther. 2020, 19, 1534735420917444. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E.; Ferrario, C.M.; Tallant, E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H2373–H2379. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mistry, H.; Kaur, G.; Aggarwal, D.; Garg, V.K.; Mittal, S.; Yerer, M.B.; Sak, K.; Khan, M.A. Gallic Acid: A Dietary Polyphenol that Exhibits Anti-neoplastic Activities by Modulating Multiple Oncogenic Targets. Anticancer Agents Med. Chem. 2022, 22, 499–514. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y.; Zhang, Z. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014, 5, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y. Effects of grape seed proanthocyanidin extract on renal injury in type 2 diabetic rats. Mol. Med. Rep. 2015, 11, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Zhang, Z.; Dai, X.; Jiang, Y.; Bao, L.; Li, Y. Grape seed proanthocyanidins ameliorate pancreatic beta-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stress. Nutr. Metab. 2013, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Dai, X.; Jiang, Y.; Zhang, Z.; Bao, L.; Li, Y.; Zhang, F.; Ma, X.; Cai, X.; Jing, L.; et al. Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 365–369. [Google Scholar] [CrossRef]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimers Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Hid, E.J.; Mosele, J.I.; Prince, P.D.; Fraga, C.G.; Galleano, M. (-)-Epicatechin and cardiometabolic risk factors: A focus on potential mechanisms of action. Pflug. Arch. 2022, 474, 99–115. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Galleano, M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: From experimental models to humans. Redox Biol. 2021, 42, 101914. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Ibarra-Lara, L.; Cuevas-Magaña, M.Y.; Sánchez-Mendoza, A.; Armada, E. Protective activities of ellagic acid and urolithins against kidney toxicity of environmental pollutants: A review. Environ. Toxicol. Pharmacol. 2022, 95, 103960. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Pirro, N.; Melo, A.C.; Tallant, E.A.; Gallagher, P. The microbiome product Urolithin A abrogates TGF-β-EGFR-PAI-1 pathway in NRK-52e renal epithelial cells. J Cell Signal. 2020, 5, 204. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chappell, M.C.; Duncan, A.V.; Melo, A.C.; Schaich, C.L.; Pirro, N.T.; Diz, D.I.; Tallant, E.A.; Gallagher, P.E. Targeted UHPLC-MS Analysis Reveals Disparate Polyphenol Composition and Concentration in Muscadine Grape Supplements with Proportional Antioxidant Activity. Antioxidants 2022, 11, 2117. https://doi.org/10.3390/antiox11112117
Chappell MC, Duncan AV, Melo AC, Schaich CL, Pirro NT, Diz DI, Tallant EA, Gallagher PE. Targeted UHPLC-MS Analysis Reveals Disparate Polyphenol Composition and Concentration in Muscadine Grape Supplements with Proportional Antioxidant Activity. Antioxidants. 2022; 11(11):2117. https://doi.org/10.3390/antiox11112117
Chicago/Turabian StyleChappell, Mark C., Aja V. Duncan, Ana Clara Melo, Christopher L. Schaich, Nancy T. Pirro, Debra I. Diz, E. Ann Tallant, and Patricia E. Gallagher. 2022. "Targeted UHPLC-MS Analysis Reveals Disparate Polyphenol Composition and Concentration in Muscadine Grape Supplements with Proportional Antioxidant Activity" Antioxidants 11, no. 11: 2117. https://doi.org/10.3390/antiox11112117