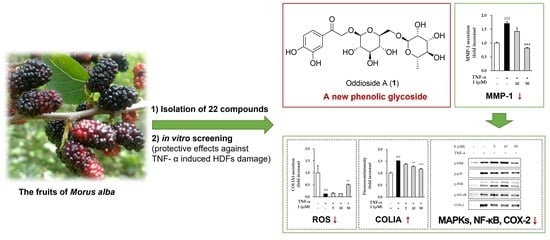
Oddioside A, a New Phenolic Glycoside Isolated from the Fruits of Morus alba (Mulberry), Protects TNF-α-Induced Human Dermal Fibroblast Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Materials
2.3. Extraction and Isolation
Oddioside A (1)
2.4. Acidic Hydrolysis of 1 and Sugar Identification
2.5. Sample Preparations
2.6. Cell Culture
2.7. Cell Viability
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. ROS Assay
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.11. Western Blotting
2.12. Statistical Analyses
3. Results
3.1. Effects of the Hot Water Extract on the Viability and MMP-1 Secretion of HDFs
3.2. Stucture Elucidation of Compound 1 and Identification of the Isolates
3.3. Effects of Compounds Isolated from the Fruits of M. alba on the Viability of HDFs
3.4. Effects of Compounds Isolated from the Fruits of M. alba on MMP-1 Secretion in TNF-α Induced HDFs
3.5. Effects of Compounds 1 on COLIA1 Protein Expression and ROS Production in TNF-α Induced HDFs
3.6. Effects of Compound 1 on Phosphorylation of MAPKs in TNF-α Induced HDFs
3.7. Effects of Compound 1 on NF-κB and COX-2 in TNF-α Treated HDFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and skin aging—From the perspective of food nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.C.M.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, H.J. Molecular mechanisms of skin aging and rejuvenation. Mol. Mech. Aging Process Rejuvenation 2016, 57–76. [Google Scholar]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Chen, S.; He, Z.; Xu, J. Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell Res. Ther. 2020, 11, 491. [Google Scholar] [CrossRef]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.-H. Protocatechuic Aldehyde Attenuates UVA-induced Photoaging in Human Dermal Fibroblast Cells by Suppressing MAPKs/AP-1 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, Y.; Meng, L.; Wu, D.; He, Y. Comparison of different CCD detectors and chemometrics for predicting total anthocyanin content and antioxidant activity of mulberry fruit using visible and near infrared hyperspectral imaging technique. Food Chem. 2017, 224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, J.Y.; Yu, J.S.; Lee, S.O.; Ryu, J.-Y.; Choi, S.-Z.; Kang, K.S.; Yamabe, N.; Kim, K.H. Odisolane, a novel oxolane derivative, and antiangiogenic constituents from the fruits of mulberry (Morus alba L.). J. Agric. Food Chem. 2016, 64, 3804–3809. [Google Scholar] [CrossRef]
- Memon, A.A.; Memon, N.; Luthria, D.L.; Bhanger, M.I.; Pitafi, A.A. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25–32. [Google Scholar]
- Khyade, V.B.; Pawar, S.S.; Khyade, R.V. Oxidative Stress Reducing capabilities of Moracin, the Novel Compound from the Fruits of Mulberry, Morus alba (L.) in Hydrogen Peroxide Induced Stress in Skin Fibroblast Cell Line Culture (AH927). Int. J. Sci. Stud. 2018, 6, 1–14. [Google Scholar]
- Shin, J.-S.; Hong, Y.; Lee, H.-H.; Ryu, B.; Cho, Y.-W.; Kim, N.-J.; Jang, D.S.; Lee, K.-T. Fulgidic acid isolated from the rhizomes of Cyperus rotundus suppresses LPS-induced iNOS, COX-2, TNF-α, and IL-6 expression by AP-1 inactivation in RAW264. 7 macrophages. Biol. Pharm. Bull. 2015, 38, 1081–1086. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, H.; Gong, Z.; Huang, J.; Pei, W.; Wang, X.; Zhang, J.; Tang, X. Antimicrobial and cytotoxic phenolics and phenolic glycosides from Sargentodoxa cuneata. Fitoterapia 2015, 101, 153–161. [Google Scholar] [CrossRef]
- Gutzeit, D.; Wray, V.; Winterhalter, P.; Jerz, G. Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophaë rhamnoides L. ssp. rhamnoides) by high-speed counter-current chromatography. Chromatographia 2007, 65, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Kikuzaki, H.; Zhu, N.; Sang, S.; Nakatani, N.; Ho, C.-T. Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.). J. Agric. Food Chem. 2000, 48, 235–238. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Festa, C.; Zollo, F.; Iorizzi, M. Phenolic glycosides from Cucumis melo var. inodorus seeds. Phytochem. Lett. 2009, 2, 130–133. [Google Scholar] [CrossRef]
- Takeda, Y.; Ooiso, Y.; Masuda, T.; Honda, G.; Otsuka, H.; Sezik, E.; Yesilada, E. Iridoid and eugenol glycosides from Nepeta cadmea. Phytochemistry 1998, 49, 787–791. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.; Pan, L.; Benatrehina, P.A.; Chai, H.; Keller, W.J.; Naman, C.B.; Kinghorn, A.D. Bioassay-guided isolation of antioxidant and cytoprotective constituents from a maqui berry (Aristotelia chilensis) dietary supplement ingredient as markers for qualitative and quantitative analysis. J. Agric. Food Chem. 2017, 65, 8634–8642. [Google Scholar] [CrossRef]
- Li, L.; Seeram, N.P. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J. Agric. Food Chem. 2010, 58, 11673–11679. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.Y.; Ling, S.K.; Chan, E.W.C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn. Res. 2014, 6, 67. [Google Scholar]
- Hattori, M. NII-electronic library service. Chem. Pharm. Bull. 2002, 27, 2091. [Google Scholar]
- Kim, D.K.; Lim, J.P.; Kim, J.W.; Park, H.W.; Eun, J.S. Antitumor and antiinflammatory constituents Fromceltis sinensis. Arch. Pharmacal Res. 2005, 28, 39–43. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 695–701. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.-J.; Yang, C.-R.; Xu, M. Phenolic antioxidants from green tea produced from Camellia crassicolumna Var. multiplex. J. Agric. Food Chem. 2009, 57, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, X.; Yu, B. Facile synthesis of flavonoid 7-O-glycosides. J. Org. Chem. 2003, 68, 6842–6845. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lundgren, L.N. Phenolics from inner bark of Pinus sylvestris. Phytochemistry 1996, 42, 1185–1189. [Google Scholar] [CrossRef]
- Walia, M.; Sharma, U.; Agnihotri, V.K.; Singh, B. Silica-supported boric acid assisted conversion of mono-and poly-saccharides to 5-hydroxymethylfurfural in ionic liquid. RSC Adv. 2014, 4, 14414–14418. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing–a morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complementary Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Hwang, E.; Gao, W.; Xiao, Y.k.; Ngo, H.T.; Yi, T.H. Helianthus annuus L. flower prevents UVB-induced photodamage in human dermal fibroblasts by regulating the MAPK/AP-1, NFAT, and Nrf2 signaling pathways. J. Cell. Biochem. 2019, 120, 601–612. [Google Scholar] [CrossRef]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective Effect of Polymethoxyflavones Isolated from Kaempferia parviflora against TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Hendra, R.; Ahmad, S.; Oskoueian, E.; Sukari, A.; Shukor, M.Y. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. BMC Complementary Altern. Med. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Gülcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complementary Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Dudonne, S.; Poupard, P.; Coutiere, P.; Woillez, M.; Richard, T.; Merillon, J.-M.; Vitrac, X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: Individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Yang, S.Y.; Cuong, N.M.; Kwon, Y.I.; Jang, H.D.; Kim, Y.H. A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and α-glucosidase inhibitory activity. Bioorganic Med. Chem. Lett. 2014, 24, 4120–4124. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Edwards, C.; Vinqvist, M.R. Media effects on antioxidant activities of phenols and catechols. J. Am. Chem. Soc. 1999, 121, 6226–6231. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; yashfa Gunawan, K.; Widowati, W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Ma, X.; Okyere, S.K.; Hu, L.; Wen, J.; Ren, Z.; Deng, J.; Hu, Y. Anti-Inflammatory Activity and Mechanism of Cryptochlorogenic Acid from Ageratina adenophora. Nutrients 2022, 14, 439. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Kapeta, S.; Chinou, I.; Vassilatou, K.; Papassideri, I.; Gonos, E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.; Kanashiro, A.; Fontanari, C.; Da Silva, E.; Lucisano-Valim, Y.; Soares, E.; Faccioli, L. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Kwon, Y.-S.; Kim, C.-M. Anti-inflammatory constituents of Polygonum bistorta. Korean J. Pharmacogn. 1999, 30, 345–349. [Google Scholar]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.m.; Zhang, H.; Liu, Z.d. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Lovell, C.; Smolenski, K.; Duance, V.; Light, N.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and other inflammatory mediators in skin aging and their inhibition by topical natural anti-inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant activity and protecting health effects of common medicinal plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lin, P.; Ngo, H.T.; Gao, W.; Wang, Y.-S.; Yu, H.-S.; Yi, T.-H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Makarov, S.S. NF-κB as a therapeutic target in chronic inflammation: Recent advances. Mol. Med. Today 2000, 6, 441–448. [Google Scholar] [CrossRef]
- Hwang, B.-M.; Noh, E.-M.; Kim, J.-S.; Kim, J.-M.; Hwang, J.-K.; Kim, H.-K.; Kang, J.-S.; Kim, D.-S.; Chae, H.-J.; You, Y.-O. Decursin inhibits UVB-induced MMP expression in human dermal fibroblasts via regulation of nuclear factor-κB. Int. J. Mol. Med. 2013, 31, 477–483. [Google Scholar] [CrossRef]
- Yang, G.; Im, H.-J.; Wang, J.H.-C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]


 ) and HMBC (
) and HMBC ( ) correlations of compound 1.
) correlations of compound 1.





| Gene | Sense Primer Sequence (5’-3’) | Antisense Primer Sequence (5’-3’) |
|---|---|---|
| MMP-1 | ATTCTACTGATATCGGGGCTTT | ATGTCCTTGGGGTATCCGTGTA |
| COLIA1 | CTCGAGGTGGACACCACCCT | CAGCTGGATGGCCACATCGG |
| β-actin | AGGAGAAGCTGTGCTACGTC | GGATGTCCACGTCACACTTC |
| Position a | 1 | |
|---|---|---|
| δH (multi J in Hz) | δC | |
| 1 | 196.8 | |
| 2a 2b | 5.14, d, 1H (17.5) 4.90, 1H, overlapped | 72.2 |
| 1′ | 128.1 | |
| 2′ | 7.42, dd, 1H (2.0) | 116.3 |
| 3′ | 146.9 | |
| 4′ | 153.1 | |
| 5′ | 6.84, d, 1H (8.5) | 115.8 |
| 6′ | 7.44, dd, 1H (8.0, 2.0) | 123.2 |
| Glc-1″ | 4.38, d, 1H (7.5) | 104.6 |
| Glc-2″ | 3.32, m | 75.2 |
| Glc-3″ | 3.44, m | 77.8 |
| Glc-4″ | 3.30, overlapped | 71.7 |
| Glc-5″ | 3.28, overlapped | 77.3 |
| Glc-6″ | 3.98, dd, 1H (11.5, 2.0) 3.63, dd, 1H (11.5, 6.5) | 68.3 |
| Rha-1‴ | 4.75, d, 1H (1.5) | 102.6 |
| Rha-2‴ | 3.68, m | 70.0 |
| Rha-3‴ | 3.86, dd, 1H (3.5, 1.5) | 72.4 |
| Rha-4‴ | 3.64, m | 72.6 |
| Rha-5‴ | 3.38, m | 74.2 |
| Rha-6‴ | 1.23, d, 3H (6.0) | 18.3 |
| TNF-α | Comp. | Conc. (µM) | MMP-1 Secretion (Fold Increase) | EC50 (µM) | Comp. | Conc. (µM) | MMP-1 Secretion (Fold Increase) | EC50 (µM) |
|---|---|---|---|---|---|---|---|---|
| − + | − − | − − | 1.00 ± 0.03 1.70 ± 0.07 | |||||
| + | 1 | 10 50 | 1.42 ± 0.12 0.82 ± 0.02 | 18.0 | 12 | 10 50 | 1.59 ± 0.04 1.09 ± 0.03 | 29.0 |
| + | 2 | 10 50 | 1.78 ± 0.00 0.89 ± 0.00 | 25.6 | 13 | 10 50 | 0.64 ± 0.04 0.55 ± 0.01 | 8.8 |
| + | 3 | 10 50 | 1.72 ± 0.00 0.96 ± 0.05 | 26.7 | 14 | 10 50 | 1.37 ± 0.07 0.95 ± 0.03 | 19.1 |
| + | 4 | 10 50 | 1.59 ± 0.04 0.82 ± 0.03 | 21.1 | 15 | 10 50 | 1.31 ± 0.04 0.99 ± 0.02 | 19.1 |
| + | 5 | 10 50 | 1.31 ± 0.04 0.93 ± 0.00 | 17.7 | 16 | 10 50 | 1.81 ± 0.03 0.79 ± 0.01 | 23.9 |
| + | 6 | 10 50 | 1.39 ± 0.00 0.89 ± 0.03 | 18.7 | 17 | 10 50 | 1.81 ± 0.03 0.98 ± 0.01 | 28.3 |
| + | 7 | 10 50 | 0.98 ± 0.04 0.76 ± 0.02 | 11.3 | 18 | 10 50 | 1.68 ± 0.08 1.02 ± 0.03 | 28.0 |
| + | 8 | 10 50 | 0.87 ± 0.04 0.25 ± 0.00 | 6.5 | 19 | 10 50 | 1.87 ± 0.01 0.71 ± 0.02 | 23.2 |
| + | 9 | 10 50 | 1.77 ± 0.10 0.70 ± 0.04 | 21.8 | 20 | 10 50 | 1.59 ± 0.00 0.83 ± 0.02 | 21.2 |
| + | 10 | 10 50 | 1.09 ± 0.03 1.00 ± 0.02 | 15.6 | 21 | 10 50 | 2.07 ± 0.07 1.07 ± 0.02 | 31.6 |
| + | 11 | 10 50 | 2.62 ± 0.04 1.01 ± 0.03 | 29.8 | 22 | 10 50 | 1.73 ± 0.11 1.13 ± 0.08 | 33.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.S.; Kim, R.; Son, S.-R.; Kang, K.S.; Jang, D.S.; Lee, S. Oddioside A, a New Phenolic Glycoside Isolated from the Fruits of Morus alba (Mulberry), Protects TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants 2022, 11, 1894. https://doi.org/10.3390/antiox11101894
Kim KS, Kim R, Son S-R, Kang KS, Jang DS, Lee S. Oddioside A, a New Phenolic Glycoside Isolated from the Fruits of Morus alba (Mulberry), Protects TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants. 2022; 11(10):1894. https://doi.org/10.3390/antiox11101894
Chicago/Turabian StyleKim, Kang Sub, Ranhee Kim, So-Ri Son, Ki Sung Kang, Dae Sik Jang, and Sullim Lee. 2022. "Oddioside A, a New Phenolic Glycoside Isolated from the Fruits of Morus alba (Mulberry), Protects TNF-α-Induced Human Dermal Fibroblast Damage" Antioxidants 11, no. 10: 1894. https://doi.org/10.3390/antiox11101894