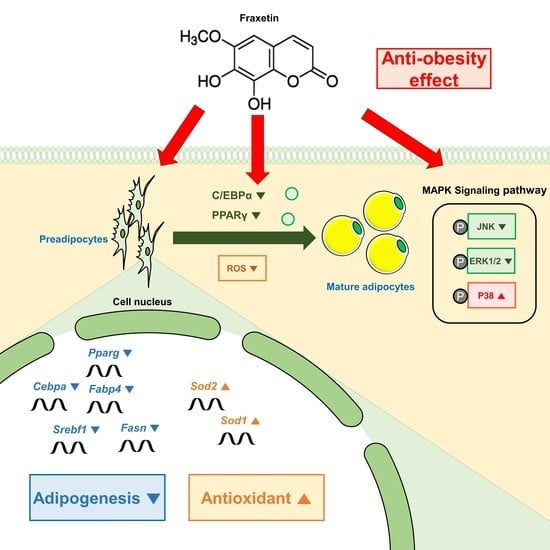
Suppressive Effect of Fraxetin on Adipogenesis and Reactive Oxygen Species Production in 3T3-L1 Cells by Regulating MAPK Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Differentiation of Preadipocytes
2.3. Cell Proliferation Test
2.4. Oil Red O Staining
2.5. Apoptosis Analysis
2.6. Cell Cycle Assay
2.7. Determination of ROS
2.8. Western Blotting
2.9. RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. Impact of Fraxetin on the Formation of Lipid Droplets and the Cell Proliferation of 3T3-L1
3.2. Effect of Fraxetin on the Activity of Differentiation-Associated Proteins and Genes
3.3. Fraxetin Inhibits Adipogenesis in the Early Stage of Differentiation
3.4. Fraxetin Alters Cell Distribution in the Cell Cycle without Apoptosis in 3T3-L1 Cells
3.5. Fraxetin Attenuates ROS Production by Activating the Expression of ROS-Scavenging Genes during the Differentiation of 3T3-L1 Cells
3.6. Fraxetin Suppresses Adipogenesis via the Regulation of MAPK Signaling
3.7. Effect of the Combination of Fraxetin and MAPK Inhibitors on Lipid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Health Organization Obesity and Overweight. WHO Newsroom Fact Sheets: 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Lavie, C.J.; Laddu, D.; Arena, R.; Ortega, F.B.; Alpert, M.A.; Kushner, R.F. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1506–1531. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; LaRose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Rhee, D.-K.; Kim, B.-O.; Pyo, S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed. Pharmacother. 2018, 102, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Jones, P.J.H. Conjugated linoleic acid and obesity control: Efficacy and mechanisms. Int. J. Obes. 2004, 28, 941–955. [Google Scholar] [CrossRef]
- Ji, E.; Jung, M.Y.; Park, J.H.; Kim, S.; Seo, C.R.; Park, K.W.; Lee, E.K.; Yeom, C.H.; Lee, S. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid. Int. J. Obes. 2013, 38, 1035–1043. [Google Scholar] [CrossRef]
- NIDDK. Prescription Medications to Treat Overweight & Obesity. 2021. Available online: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 27 July 2022).
- Guerciolini, R. Mode of action of orlistat. Int. J. Obes. 1997, 21, S12–S23. [Google Scholar]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Moon, H.-S.; Chung, C.-S.; Lee, H.-G.; Kim, T.-G.; Choi, Y.-J.; Cho, C.-S. Inhibitory Effect of (−)-Epigallocatechin-3-Gallate on Lipid Accumulation of 3T3-L1 Cells. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Kajimoto, Y.; Watada, H.; Kaneto, H.; Kishimoto, M.; Umayahara, Y.; Fujitani, Y.; Kamada, T.; Kawamori, R.; Yamasaki, Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J. Clin. Investig. 1997, 99, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Jang, H.-D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int. J. Mol. Sci. 2021, 22, 6096. [Google Scholar] [CrossRef]
- Wu, C.-R.; Huang, M.-Y.; Lin, Y.-T.; Ju, H.-Y.; Ching, H. Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Liu, R.; Sun, Q.; Sun, A.; Cui, J. Isolation and purification of coumarin compounds from Cortex fraxinus by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1072, 195–199. [Google Scholar] [CrossRef]
- Wang, H.; Zou, D.; Xie, K.; Xie, M. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014, 10, 2341–2345. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Yan, Y.; Wang, H. Effect of fraxetin on proliferation and apoptosis in breast cancer cells. Oncol. Lett. 2017, 14, 7374–7378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.; Choi, K.-M.; Yoo, H.-S.; Lee, C.-K.; Hwang, B.Y.; Lee, M.K. Inhibitory Effects of Coumarins from the Stem Barks of Fraxinus rhynchophylla on Adipocyte Differentiation in 3T3-L1 Cells. Biol. Pharm. Bull. 2010, 33, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Song, G.; Bae, H. Matairesinol Induces Mitochondrial Dysfunction and Exerts Synergistic Anticancer Effects with 5-Fluorouracil in Pancreatic Cancer Cells. Mar. Drugs 2022, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Park, B.O.; Ahrends, R.; Teruel, M.N. Consecutive Positive Feedback Loops Create a Bistable Switch that Controls Preadipocyte-to-Adipocyte Conversion. Cell Rep. 2012, 2, 976–990. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell Cycle and Apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Sharma, A.M.; Chetty, V.T. Obesity, hypertension and insulin resistance. Acta Diabetol. 2005, 42 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Wells, J.C. The evolution of human adiposity and obesity: Where did it all go wrong? Dis. Model. Mech. 2012, 5, 595–607. [Google Scholar] [CrossRef]
- Auwerx, J.; Martin, G.; Guerre-Millo, M.; Staels, B. Transcription, adipocyte differentiation, and obesity. J. Mol. Med. 1996, 74, 347–352. [Google Scholar] [CrossRef]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.; Hocht, C.; Puyo, A.; Taira, C. Recent Advances in Obesity Pharmacotherapy. Curr. Clin. Pharmacol. 2009, 4, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Yao, Z.-L.; Yang, D.; Ke, J.; Wu, Q.-L.; Li, J.-K.; Zhou, X.-D. Chemical Constituents from Fraxinus hupehensis and Their Antifungal and Herbicidal Activities. Biomolecules 2020, 10, 74. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARγ Expression and/or Its Transcriptional Activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding Adipocyte Differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Jiang, M.S.; Lane, M.D. Repressive effect of Sp1 on the C/EBPalpha gene promoter: Role in adipocyte differentiation. Mol. Cell. Biol. 1999, 19, 4855–4865. [Google Scholar] [CrossRef]
- Schadinger, S.E.; Bucher, N.L.; Schreiber, B.M.; Farmer, S.R. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1195–E1205. [Google Scholar] [CrossRef]
- Lakshmanan, M.R.; Nepokroeff, C.M.; Porter, J.W. Control of the Synthesis of Fatty-Acid Synthetase in Rat Liver by Insulin, Glucagon, and Adenosine 3′:5′ Cyclic Monophosphate. Proc. Natl. Acad. Sci. USA 1972, 69, 3516–3519. [Google Scholar] [CrossRef]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef]
- Ayala-Sumuano, J.-T.; Velez-Delvalle, C.; Beltrán-Langarica, A.; Marsch-Moreno, M.; Cerbón-Solorzano, J.; Kuri-Harcuch, W. Srebf1a is a key regulator of transcriptional control for adipogenesis. Sci. Rep. 2011, 1, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, S.-Y.; Chen, Y.-C.; Tsai, Y.-C.; Hung, Y.-W.; Hsu, C.-H.; Lee, Y.M.; Cheng, P.-Y. Raspberry Ketone Reduced Lipid Accumulation in 3T3-L1 Cells and Ovariectomy-Induced Obesity in Wistar Rats by Regulating Autophagy Mechanisms. J. Agric. Food Chem. 2017, 65, 10907–10914. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.; Lee, S.-H.; Ko, H.M.; Jung, J.H.; Chung, W.-S.; Jang, H.-J. Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 10405. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chou, G. Isoflavonoids from Crotalaria albida Inhibit Adipocyte Differentiation and Lipid Accumulation in 3T3-L1 Cells via Suppression of PPAR-γ Pathway. PLoS ONE 2015, 10, e0135893. [Google Scholar] [CrossRef]
- You, L.; Li, F.; Sun, Y.; Luo, L.; Qin, J.; Wang, T.; Liu, Y.; Lai, R.; Li, R.; Guo, X.; et al. Extract of Acalypha australis L. inhibits lipid accumulation and ameliorates HFD-induced obesity in mice through regulating adipose differentiation by decreasing PPARγ and CEBP/α expression. Food Nutr. Res. 2021, 65, 10. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Camacho, C.; Beltrán-Langarica, A.; Ochoa-Uribe, A.K.; Marsch-Moreno, M.; Ayala-Sumuano, J.T.; Velez-delValle, C.; Kuri-Harcuch, W. The transient expression of Klf4 and Klf5 during adipogenesis depends on GSK3β activity. Adipocyte 2015, 4, 248–255. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 2006, 55, 928–934. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Aouadi, M.; Laurent, K.; Prot, M.; Le Marchand-Brustel, Y.; Binétruy, B.; Bost, F. Inhibition of p38MAPK Increases Adipogenesis From Embryonic to Adult Stages. Diabetes 2006, 55, 281–289. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwon, S.Y.; Ahn, J.Y.; Jung, C.H.; Moon, B.K.; Ha, T.Y. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complement. Altern. Med. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, N.; Phillips, B.W.; Massiera, F.; Villageois, P.; Wdziekonski, B.; Saint-Marc, P.; Nichols, J.; Aubert, J.; Saeki, K.; You, A.; et al. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol. Endocrinol. 2001, 15, 2037–2049. [Google Scholar] [PubMed]
- Aouadi, M.; Jager, J.; Laurent, K.; Gonzalez, T.; Cormont, M.; Binétruy, B.; Brustel, Y.L.M.; Tanti, J.-F.; Bost, F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007, 581, 5591–5596. [Google Scholar] [CrossRef]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific Inhibitors of p38 Mitogen-activated Protein Kinase Block 3T3-L1 Adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef]
- Hata, K.; Nishimura, R.; Ikeda, F.; Yamashita, K.; Matsubara, T.; Nokubi, T.; Yoneda, T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol. Biol. Cell. 2003, 14, 545–555. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [Green Version]








| Chemicals | Catalog Number | Supplier |
|---|---|---|
| p-JNK (Thr183/Tyr185) | 4668 | Cell Signaling Technology (Beverly, MA, USA) |
| JNK | 9252 | Cell Signaling Technology |
| p-ERK1/2 (Thr202/Tyr204) | 9101 | Cell Signaling Technology |
| ERK1/2 | 4695 | Cell Signaling Technology |
| p-P38 (Thr180/Tyr182) | 4511 | Cell Signaling Technology |
| P38 | 9212 | Cell Signaling Technology |
| PPARγ | 2443 | Cell Signaling Technology |
| C/EBPα | 8178 | Cell Signaling Technology |
| β-actin | sc-47778 | Santa Cruz Biotechnology (CA, USA) |
| SB203580 | BML-EI286 | Enzo Life Sciences (Farmingdale, NY) |
| U0126 | BML-EI282 | Enzo Life Sciences |
| SP600125 | BML-EI305 | Enzo Life Sciences |
| Gene | Size (bp) | GenBank Accession No. | Primer Sequence (5’→3’) |
|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Pparg) | 126 | NM_001127330.2 | F: CGAGTCTGTGGGGATAAAGC |
| R: CCGGCAGTTAAGATCACACC | |||
| CCAAT enhancer binding protein alpha (Cebpa) | 87 | NM_001287514.1 | F: GGTGGACAAGAACAGCAACG |
| R: CGTTGTTTGGCTTTATCTCG | |||
| Fatty-acid-binding protein 4 (Fabp4) | 131 | NM_024406.3 | F: GCCCAACATGATCATCAGC |
| R: TCACCTTCCTGTCGTCTGC | |||
| Fatty acid synthase (Fasn) | 95 | NM_007988.3 | F: AGCACACATCCTAGGCATCC |
| R: GAACTTCCACACCCATGAGC | |||
| Sterol regulatory-element-binding transcription factor 1 (Srebf1) | 146 | NM_001313979.1 | F: AGGTCACCGTTTCTTTGTGG |
| R: AGTTCAACGCTCGCTCTAGG | |||
| Kruppel-like factor 5 (Klf5) | 127 | NM_009769.4 | F: ACAAATCCCAGAGACCATGC |
| R: CTAGTGAACTCGGGGAGAGC | |||
| Superoxide dismutase 1 (Sod1) | 87 | NM_011434.2 | F: CAAGATGACTTGGGCAAAGG |
| R: AATCCCAATCACTCCACAGG | |||
| Superoxide dismutase 2 (Sod2) | 81 | NM_013671.3 | F: GTGTCTGTGGGAGTCCAAGG |
| R: GCAGGCAGCAATCTGTAAGC | |||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 130 | NM_001289726.1 | F: AACTTTGGCATTGTGGAAGG |
| R: ATGCAGGGATGATGTTCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.; Song, G.; Bae, H. Suppressive Effect of Fraxetin on Adipogenesis and Reactive Oxygen Species Production in 3T3-L1 Cells by Regulating MAPK Signaling Pathways. Antioxidants 2022, 11, 1893. https://doi.org/10.3390/antiox11101893
Lee W, Song G, Bae H. Suppressive Effect of Fraxetin on Adipogenesis and Reactive Oxygen Species Production in 3T3-L1 Cells by Regulating MAPK Signaling Pathways. Antioxidants. 2022; 11(10):1893. https://doi.org/10.3390/antiox11101893
Chicago/Turabian StyleLee, Woonghee, Gwonhwa Song, and Hyocheol Bae. 2022. "Suppressive Effect of Fraxetin on Adipogenesis and Reactive Oxygen Species Production in 3T3-L1 Cells by Regulating MAPK Signaling Pathways" Antioxidants 11, no. 10: 1893. https://doi.org/10.3390/antiox11101893