A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.)
Abstract
:1. Introduction
2. Materials and Methods
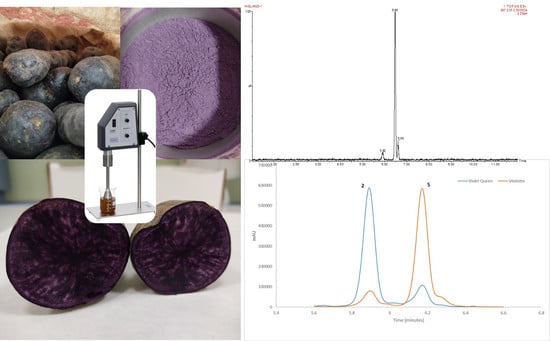
2.1. Biological Material
2.2. Chemical and Solvents
2.3. Ultrasound-Assisted Extraction
2.3.1. Ultrasound-Assisted Extraction Equipment
2.3.2. Ultrasound-Assisted Extraction Optimization
2.4. Identification of Anthocyanins by UHPLC-PDA-QToF-MS
2.5. Separations and Quantification of the Anthocyanins by UHPLC–UV–Vis
2.6. DPPH Analysis
2.7. Optimization Study
3. Results and Discussion
3.1. Optimization of the Method
3.2. Optimal Extraction Time of the Method
3.3. Repeatability and Intermediate Precision
3.4. Re-Extraction Analysis
3.5. Applying the Optimized Method to Different Potato Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pęksa, A.; Miedzianka, J.; Nemś, A.; Rytel, E. The free-amino-acid content in six potatoes cultivars through storage. Molecules 2021, 26, 1322. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Guo, K.; Liu, T.; Bian, X.; Zhang, L.; Wei, C. Effects of different isolation media on structural and functional properties of starches from root tubers of purple, yellow and white sweet potatoes. Molecules 2018, 23, 2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguerol, A.T.; Igual, M.; Pagán-Moreno, M.J. Nutritional, physico-chemical and mechanical characterization of vegetable fibers to develop fiber-based gel foods. Foods 2021, 10, 1017. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging processing technologies for the recovery of valuable bioactive compounds from potato peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Akyıldız, A.; Polat, S.; Agçam, E.; Fenercioglu, H. Potato and potato-processing technology. In Handbook of Vegetable Preservation and Processing; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 591–594. [Google Scholar]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic compounds and antioxidant activities of potato cultivars with white, yellow, red and purple flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, C.A.; Wechsler, L.J.; Morrissey, M.T. Using a fractional model to measure the impact of antioxidant information, price, and liking on purchase intent for specialty potatoes. Food Qual. Prefer. 2015, 46, 66–78. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, D.; Song, X.; Deng, Y.; Zhao, Y. Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Res. Int. 2018, 105, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264. 7 macrophages. Food Res. Int. 2020, 137, 109647. [Google Scholar] [CrossRef]
- Torres, A.; Noriega, L.G.; Delgadillo-Puga, C.; Tovar, A.R.; Navarro-Ocaña, A. Caffeoylquinic acid derivatives of purple sweet potato as modulators of mitochondrial function in mouse primary hepatocytes. Molecules 2021, 26, 319. [Google Scholar] [CrossRef]
- Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and antioxidative potential of the blue congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Pan, Z.; Yang, C.; Jia, Z.; Guo, X. Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea Batatas) storage roots. Molecules 2019, 24, 4476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Chae, J.; Kim, D.S.; Lee, J.-B.; Kwon, G.-S.; Kwon, T.K.; Nam, J.-O. Enhancement of the antiobesity and antioxidant effect of purple sweet potato extracts and enhancement of the effects by fermentation. Antioxidants 2021, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Sampaio, S.L.; Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Ferreira, I. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part I: Root vegetables. Antioxidants 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Kostyn, K.; Boba, A.; Kostyn, A.; Kozak, B.; Starzycki, M.; Kulma, A.; Szopa, J. Expression of the tyrosine hydroxylase gene from rat leads to oxidative stress in potato plants. Antioxidants 2020, 9, 717. [Google Scholar] [CrossRef]
- Im, Y.R.; Kim, I.; Lee, J. Phenolic composition and antioxidant activity of purple sweet potato (Ipomoea Batatas (L.) Lam.): Varietal comparisons and physical distribution. Antioxidants 2021, 10, 462. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical characterization of five edible purple-reddish vegetables: Anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Du, M.; Navarre, D.A.; Zhu, M. Effect of cooking methods on bioactivity of polyphenols in purple potatoes. Antioxidants 2021, 10, 1176. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of dietary anthocyanins on biomarkers of oxidative stress and antioxidative capacity: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2020, 68, 103912. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- Li, G.; Lin, Z.; Zhang, H.; Liu, Z.; Xu, Y.; Xu, G.; Li, H.; Ji, R.; Luo, W.; Qiu, Y.; et al. Anthocyanin accumulation in the leaves of the purple sweet potato (Ipomoea Batatas L.) cultivars. Molecules 2019, 24, 3743. [Google Scholar] [CrossRef] [Green Version]
- Aliaño-González, M.J.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Ayuso, J.; Álvarez, J.Á.; Barbero, G.F. Extraction of anthocyanins and total phenolic compounds from Açai (Euterpe Oleracea Mart.) using an experimental design methodology. Part 2: Ultrasound-assisted extraction. Agronomy 2020, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.Á.; Barbero, G.F.; Ayuso, J. Optimization of analytical ultrasound-assisted methods for the extraction of total phenolic compounds and anthocyanins from sloes (Prunus Spinosa L.). Agronomy 2020, 10, 966. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Álvarez, J.Á.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a novel method based on ultrasound-assisted extraction for the quantification of anthocyanins and total phenolic compounds in blueberry samples (Vaccinium Corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Giusti, M.M. Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 2020, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Carrera, C.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid UHPLC-PDA method for the simultaneous quantification of flavonol contents in onions (Allium Cepa L.). Pharmaceuticals 2021, 14, 310. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Ereminas, G.; Majiene, D.; Sidlauskas, K.; Jakstas, V.; Ivanauskas, L.; Vaitiekaitis, G.; Liobikas, J. Neuroprotective properties of anthocyanidin glycosides against H2O2-induced glial cell death are modulated by their different stability and antioxidant activity in vitro. Biomed. Pharmacother. 2017, 94, 188–196. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Antioxidant capacity of anthocyanins and other vegetal pigments. Antioxidants 2020, 9, 665. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Han, Y.; Guo, Y.; Cui, S.W.; Li, H.; Shan, Y.; Wang, H. Purple sweet potato extract extends lifespan by activating autophagy pathway in male drosophila melanogaster. Exp. Gerontol. 2021, 144, 111190. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, Z.; Zhang, B.; Chen, J.; Liu, R.; Song, D.; Li, W.; Lin, N.; Zou, X.; Wang, J.; et al. Purification, characterization, and in vitro antitumor activity of a novel glucan from the purple sweet potato Ipomoea Batatas (L.) Lam. Carbohydr. Polym. 2021, 257, 117605. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of Andean blueberry in bioactive compounds, evaluation of biological properties, and in vitro bioaccessibility. Foods 2020, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe Oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of anthocyanin extracted from purple flesh cultivated potatoes by spray drying and its effects on in vitro gastrointestinal digestion. Molecules 2020, 25, 722. [Google Scholar] [CrossRef] [Green Version]
- Ercoli, S.; Parada, J.; Bustamante, L.; Hermosín-Gutiérrez, I.; Contreras, B.; Cornejo, P.; Ruiz, A. Noticeable quantities of functional compounds and antioxidant activities remain after cooking of colored fleshed potatoes native from Southern Chile. Molecules 2021, 26, 314. [Google Scholar] [CrossRef]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.; Yang, B. Impact of cultivar, growth temperature and developmental stage on phenolic compounds and ascorbic acid in purple and yellow potato tubers. Food Chem. 2020, 326, 126966. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- De Masi, L.; Bontempo, P.; Rigano, D.; Stiuso, P.; Carafa, V.; Nebbioso, A.; Piacente, S.; Montoro, P.; Aversano, R.; D’Amelia, V.; et al. Comparative phytochemical characterization, genetic profile, and antiproliferative activity of polyphenol-rich extracts from pigmented tubers of different solanum tuberosum varieties. Molecules 2020, 25, 233. [Google Scholar] [CrossRef] [Green Version]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.A.; Barbero, G.F.; Ayuso, J. Extraction of antioxidants from blackberry (Rubus Ulmifolius L.): Comparison between ultrasound- and microwave-assisted extraction techniques. Agronomy 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Aourach, M.; González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Essalmani, H.; Palma, M.; Barbero, G.F. Optimization and comparison of ultrasound and microwave-assisted extraction of phenolic compounds from cotton-lavender (Santolina Chamaecyparissus L.). Agronomy 2021, 11, 84. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and comparing ultrasound- and microwave-assisted extraction methods applied to the extraction of antioxidant capsinoids in peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, T.; He, J.; Barba, F.; Cravotto, G.; Koubaa, M. Ultrasound-assisted extraction, centrifugation and ultrafiltration: Multistage process for polyphenol recovery from purple sweet potatoes. Molecules 2016, 21, 1584. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced recovery of phenolic and tocopherolic compounds from walnut (Juglans Regia L.) male flowers based on process optimization of ultrasonic assisted-extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-assisted extraction and natural deep eutectic solvents combination: A green strategy to improve the recovery of phenolic compounds from lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Z.; Ma, S.; Rasool, M.A.; Wang, L.; Zhang, J. Optimization of ultrasonic-assisted extraction, refinement and characterization of water-soluble polysaccharide from Dictyosphaerium sp. and evaluation of antioxidant activity in vitro. J. Food Meas. Charact. 2020, 14, 963–977. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.R.; Iglesias, A.H.; Martínez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2021, 67, 102549. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Majid, H.; Silva, F.V.M. Kanuka bush leaves for Alzheimer’s disease: Improved inhibition of β-secretase enzyme, antioxidant capacity and yield of extracts by ultrasound assisted extraction. Food Bioprod. Process. 2021, 128, 109–120. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Celotti, E.; Stante, S.; Ferraretto, P.; Román, T.; Nicolini, G.; Natolino, A. High power ultrasound treatments of red young wines: Effect on anthocyanins and phenolic stability indices. Foods 2020, 9, 1344. [Google Scholar] [CrossRef]
- Antoniou, C.; Kyratzis, A.; Rouphael, Y.; Stylianou, S.; Kyriacou, M.C. Heat- and ultrasound-assisted aqueous extraction of soluble carbohydrates and phenolics from carob kibbles of variable size and source material. Foods 2020, 9, 1364. [Google Scholar] [CrossRef]
- Jiménez, N.; Bassama, J.; Bohuon, P. Estimation of the kinetic parameters of anthocyanins degradation at different water activities during treatments at high temperature (100–140 °C) using an unsteady-state 3D model. J. Food Eng. 2020, 279, 109951. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Thirugnanasambandham, K.; Nivetha, C.V.; Dinesh, R. Box-behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of ultrasound assisted extraction as an alternative method for the extraction of anthocyanins and total phenolic compounds from maqui berries (Aristotelia Chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid and accurate UHPLC-PDA-FL method for the quantification of phenolic compounds in grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef] [PubMed]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol composition and antioxidant activity of onions (Allium Cepa L.) based on the development of new analytical ultrasound-assisted extraction methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-assisted extraction of two types of antioxidant compounds (TPC and TA) from black chokeberry (Aronia Melanocarpa L.): Optimization of the individual and simultaneous extraction methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Kita, A.; Bakowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum Tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Carrera, C.; Aliaño-González, M.J.; Rodríguez-López, J.; Ferreiro-González, M.; Ojeda-Copete, F.; Barbero, G.F.; Palma, M. Optimization of an ultrasound-assisted extraction method for the analysis of major anthocyanin content in Erica australis flowers. Molecules 2021, 26, 2884. [Google Scholar] [CrossRef] [PubMed]
- Mariychuk, R.; Eliasova, A.; Porubska, J.; Poracova, J.; Simko, V. Isolation and lyophilisation of anthocyanins from fruits of blackcurrant. Acta Hortic. 2016, 1133, 329–333. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Kita, A.; Bakowska-Barczak, A.; Lisińska, G.; Hamouz, K.; Kułakowska, K. Antioxidant activity and quality of red and purple flesh potato chips. LWT Food Sci. Technol. 2015, 62, 525–531. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvořák, P.; Čepl, J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]




| Experiment | % MeOH | Temperature | pH | Ratio | Relative Area (Measured) | Relative Area (Predicted) | Relative Error in the Prediction (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | −1 | 0 | −1 | 72,953.22 | 74,783.20 | 2.45 |
| 2 | 1 | 0 | 0 | −1 | 188,615.38 | 173,546.00 | 8.68 |
| 3 | 1 | 0 | 0 | 1 | 205,339.62 | 197,649.00 | 3.89 |
| 4 | 0 | 0 | 0 | 0 | 98,233.05 | 114,049.00 | 13.87 |
| 5 | 0 | 1 | 0 | −1 | 209,930.07 | 209,546.00 | 0.18 |
| 6 | 0 | 0 | −1 | 1 | 24,3104.45 | 263,197.00 | 7.63 |
| 7 | 0 | 0 | 1 | −1 | 142,070.86 | 143,175.00 | 0.77 |
| 8 | 1 | 0 | 1 | 0 | 197,433.36 | 182,238.00 | 8.34 |
| 9 | 1 | −1 | 0 | 0 | 120,781.49 | 127,148.00 | 5.01 |
| 10 | 0 | 1 | −1 | 0 | 324,735.63 | 330,203.00 | 1.66 |
| 11 | 0 | 0 | 1 | 1 | 97,182.43 | 109,982.00 | 11.64 |
| 12 | −1 | 0 | −1 | 0 | 64,754.72 | 72,322.10 | 10.46 |
| 13 | 0 | 1 | 1 | 0 | 255,505.62 | 247,362.00 | 3.29 |
| 14 | −1 | 0 | 0 | −1 | 33,589.36 | 32,288.70 | 4.03 |
| 15 | −1 | 1 | 0 | 0 | 68,379.59 | 63,210.00 | 8.18 |
| 16 | 0 | 0 | −1 | −1 | 187,493.78 | 195,891.00 | 4.29 |
| 17 | 0 | −1 | −1 | 0 | 236,810.85 | 201,385.00 | 17.59 |
| 18 | 1 | 1 | 0 | 0 | 348,856.93 | 316,545.00 | 10.21 |
| 19 | 0 | −1 | 0 | 1 | 74,903.66 | 77,659.60 | 3.55 |
| 20 | 0 | −1 | 1 | 0 | 77,330.88 | 78,294.80 | 1.23 |
| 21 | −1 | 0 | 1 | 0 | 376.90 | 347.63 | 8.42 |
| 22 | 1 | 0 | −1 | 0 | 282,293.09 | 316,194.00 | 10.72 |
| 23 | −1 | −1 | 0 | 0 | 41,213.04 | 45,278.10 | 8.98 |
| 24 | 0 | 0 | 0 | 0 | 116,928.90 | 114,049.00 | 2.53 |
| 25 | 0 | 0 | 0 | 0 | 96,983.88 | 114,049.00 | 14.96 |
| 26 | 0 | 1 | 0 | 1 | 260,240.38 | 240,782.00 | 8.08 |
| 27 | −1 | 0 | 0 | 1 | 26,220.55 | 22,278.80 | 17.69 |
| Variable | Sum of Squares | F Value | p-Value |
|---|---|---|---|
| %MeOH | 1.36 × 1011 | 41.92 | 0.00 |
| Temperature | 6.66 × 1010 | 20.52 | 0.00 |
| pH | 3.18 × 1010 | 9.81 | 0.01 |
| Ratio | 8.73 × 108 | 0.27 | 0.61 |
| %MeOH*%MeOH | 6.57 × 109 | 2.02 | 0.18 |
| %MeOH*Temperature | 1.64 × 109 | 0.50 | 0.49 |
| %MeOH*pH | 9.60 × 108 | 0.30 | 0.60 |
| %MeOH*Ratio | 4.97 × 107 | 0.02 | 0.90 |
| Temperature*Temperature | 7.08 × 109 | 2.18 | 0.17 |
| Temperature*pH | 4.05 × 108 | 0.12 | 0.73 |
| Temperature*Ratio | 2.01 × 108 | 0.06 | 0.81 |
| pH*pH | 2.17 × 1010 | 6.70 | 0.02 |
| pH*Ratio | 2.53 × 109 | 0.78 | 0.39 |
| Ratio*Ratio | 2.07 × 105 | 0.00 | 0.99 |
| Error total | 3.24 × 109 |
| Repeatability | Intermediate Precision | |
|---|---|---|
| Average | 304,245.41 | 297,867.39 |
| SD * | 8012.67 | 10,154.10 |
| RSD ** | 2.63 | 3.41 |
| Compound (mg/g DW)/Variety | Vitelotte | Double Fun | Violet Queen | Highland |
|---|---|---|---|---|
| 1 | n.d. | n.d. | n.d. | 0.14 ± 0.00 |
| 2 | 0.66 ± 0.05 a | 0.59 ± 0.02 a | 3.82 ± 0.10 b | n.d. |
| 3 | n.d. | n.d. | n.d. | 1.12 ± 0.01 |
| 4 | n.d. | n.d. | 0.14 ± 0.01 | n.d. |
| 5 | 3.97 ± 0.14 c | 1.98 ± 0.09 b | 0.91 ± 0.09 a | n.d. |
| 6 | n.d. | n.d. | n.d. | 0.22 ± 0.00 |
| 7 | 0.34 ± 0.08 b | 0.21 ± 0.03 a | n.d. | n.d. |
| Total anthocyanins (mg/g) | 4.97 ± 0.17 c | 2.78 ± 0.10 b | 4.87 ± 0.13 c | 1.48 ± 0.01 a |
| DPPH (mg TE/g DW) | 28.81 | 21.67 | 29.87 | 11.19 |
| Flesh Color | Purple | Purple/White | Purple | Red |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera, C.; Aliaño-González, M.J.; Valaityte, M.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.). Antioxidants 2021, 10, 1375. https://doi.org/10.3390/antiox10091375
Carrera C, Aliaño-González MJ, Valaityte M, Ferreiro-González M, Barbero GF, Palma M. A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.). Antioxidants. 2021; 10(9):1375. https://doi.org/10.3390/antiox10091375
Chicago/Turabian StyleCarrera, Ceferino, María José Aliaño-González, Monika Valaityte, Marta Ferreiro-González, Gerardo F. Barbero, and Miguel Palma. 2021. "A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.)" Antioxidants 10, no. 9: 1375. https://doi.org/10.3390/antiox10091375