Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans
Abstract
:1. Introduction
2. Search Strategy
3. Content, Structure, and Metabolism of Isoflavones
3.1. Isoflavone Content in Soybean
3.2. Isoflavone Biosynthesis and Regulation
3.3. Structure and Type of Isoflavones
3.4. Functionality of Isoflavones in Plants
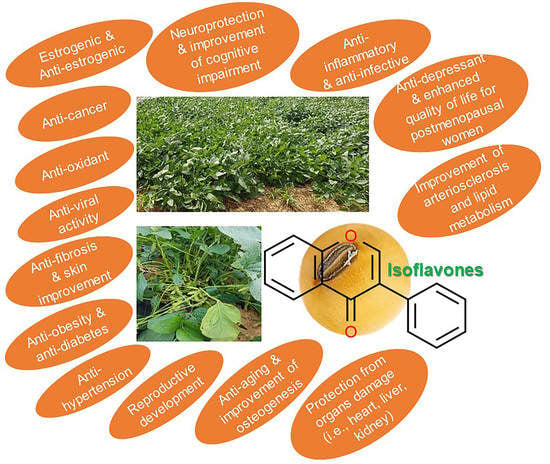
4. The Role of Isoflavones in Humans
4.1. Absorption and Metabolism
4.2. Estrogen-Like Effect and Activity
4.3. Neuroprotective Effect and Improvement of Cognitive Impairment
4.4. Antidepressant Effects
4.5. Anti-Obesity Effects
4.6. Effects on Improving Metabolic Syndrome
4.7. Improvements of Blood Pressure
4.8. Improvement of Cardiac Function
4.9. Protection against Liver Damage
4.10. Improvement of Renal Dysfunction

4.11. Anti-Inflammatory Effects
4.12. Anticarcinogenic Activity through Gene Regulation
4.13. Promotion of Osteogenesis and the Prevention of Osteoporosis
4.14. Improvement of Blood Glucose Homeostasis
4.15. Reduced Inflammation and Skin Cell Protection in Wound Tissue
4.16. Enhanced Quality of Life for Postmenopausal Women Based on Improved Physical and Emotional Symptoms

4.17. Safety of Isoflavones in Reproductive Development
4.18. Other Human Beneficial Effects of Isoflavones
4.18.1. Improvement of Arteriosclerosis and Lipid Metabolism
4.18.2. Antioxidant Activity
4.18.3. Antiviral Effect
4.18.4. Allergy Relief
4.19. Other Considerations
4.20. Side Effects
5. Conclusions
- (1)
- Isoflavones can activate the estrogen receptor and may function as an estrogen or an antiestrogen depending on the physiological conditions or chemical structures.
- (2)
- The anticancer activity of isoflavones comes mostly from genistein which is an antiestrogen that weakly binds to the estrogen receptor. Genistein reduces cancer cell proliferation and promotes the division of normal cells. Genistein also inhibits angiogenesis to block the supply of oxygen or nutrients, thereby suppressing tumor development.
- (3)
- Isoflavones in vitro and in vivo show antioxidant effects equivalent those of vitamins E and C.
- (4)
- The estrogen effect of isoflavones can be utilize to improve the physical and emotional symptoms in post-menopausal women.
- (5)
- While the animal protein casein increases plasma cholesterol levels, soy isoflavone decreases them, indicating its potential use as a cholesterol lowering agent.
- (6)
- Isoflavones prevent calcium leaks from the bones by elevating vitamin D activity that enhances calcium absorption, thus lowering the risk of osteoporosis.
- (7)
- The reported effects of isoflavones include reducing blood pressure, enhancing the effects of lipid metabolism, anti-mutational effects, antimicrobial activity, antiviral activity, and skin protective effects.
6. Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ABC | ATP-binding cassette |
| AC | adenylyl cyclase |
| ACC | acetyl-CoA carboxylase |
| ADRP | adipose differentiation-related protein activator protein 1 |
| AhR | aryl hydrocarbon receptor |
| AICD | APP intracellular domain |
| Akt | protein kinase B (serine/threonine-specific protein kinase) |
| ALP | alkaline phosphatase |
| AMPK | 5′-adenosine monophosphate-activated protein kinase |
| ANS | anthocyanidin synthase |
| AP1 | activator protein 1 |
| APP | amyloid precursor protein |
| AR | androgen receptor |
| ARE | antioxidant responsive element |
| AT | acetyltransferase |
| ATF2 | activating transcription factor 2 ATP, adenosine triphosphate |
| BAD | Bcl2-associated agonist of cell death |
| B.Ar | bone area |
| BCA | biochanin A |
| BMI | body mass index |
| BMP2 | bone morphogenetic protein 2 |
| BV/TV | bone volume/tissue volume |
| Ca2+ | calcium ion |
| C/EBP | CCAAT/enhancer-binding proteins |
| CaMK II | calmodulin kinase II |
| cAMP | cyclic adenosine monophosphate |
| C/EBP-α | CCAAT/enhancer-binding protein-alpha |
| c-Fos | cellular oncogene fos |
| C4H | cinnamic acid 4-hydroxylae |
| CHI | chalcone isomerase |
| CHR | chalcone reductase |
| CHS | chalcone synthase |
| 4CL | 4-coumarate-CoA ligase |
| COL1a1 | transcription factor Sp7 (Osterix), alpha-1 type I collagen |
| COX-2 | cyclooxygenase 2 |
| CRE | carbohydrate response element |
| CREBp | cyclic AMP-responsive element-binding protein |
| CTF | C-terminal fragment |
| CTSK | cathepsin K |
| DFR | dihydroxyflavonol reductase |
| DMID | 7,2′-dihydroxy-4′-methoxy-isoflavanol dehydratase |
| DHD | dihydrodaidzein |
| DR5 | tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 |
| DZ | daidzein |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| eNOS | endothelial nitric oxide synthase |
| EPR | endoplasmic reticulum |
| ER | estrogen receptor |
| ERAα/β | estrogen receptor A alpha/beta |
| ERα | estrogen receptor alpha |
| ERβ | estrogen receptor beta |
| ERK1/2 | extracellular signal-regulated kinase 1 and 2 |
| F3H | flavanone 3-hydroxylase |
| FADD | Fas-associated protein with death domain |
| FAS | fatty acid synthetase |
| FASL (CD95L) | FAS ligand |
| PARP | poly(ADP-ribose)-polymerase |
| FBG | fasting blood glucose |
| FFAs | free fatty acids |
| FLS | flavonol synthase |
| FNS | flavone synthase |
| FNT | formononetin |
| FOXO | forkhead box transcription factor family O |
| G2′DT | glycinol 2-dimethylallyltransferase |
| G4′DT | glycinol 4-dimethylallyltransferase |
| G6Pase | glucose-6-phosphatase |
| GC | glycetin |
| GDNF | glial cell line-derived neurotrophic factor |
| GFr | growth factor receptor |
| GK | glucokinase |
| GLS | glyceollin synthase |
| GLUT4 | glucose transporter type 4 |
| GPX | glutathione peroxidase |
| gp91 (NOX2) | NADPH oxidase subunit |
| G or GS | genistein |
| GSH | glutathione (reduced form) |
| GSK3β | glycogen synthase kinase 3 beta |
| GT | glucosyltransferase |
| 6-HD | 6-hydroxydaidzein |
| 8-HD | 8-hydroxydaidzein |
| 12′H | isoflavone hydroxylase |
| HER2 | human epidermal growth factor receptor 2 |
| HI4′OMT | 2,7,4′-trihydroxyisoflavanone 4′-O-methyltransferase |
| HID | 2-hydroxyisoflavone dehydratase |
| 2-HIS | 2-hydroxyisoflavone synthase |
| HNF4α | hepatocyte nuclear factor 4 alpha |
| HO-1 | heme oxygenase 1 |
| I2′H | isoflavone 2-hydroxylase |
| ICAM-1 | intracellular adhesion molecule 1 |
| ICHG | isoflavone conjugate-hydrolyzing β-glucosidase |
| IF | isoflavone |
| IF7GT | isoflavone-7-O-glucosyltransferase |
| IF7MaT | isoflavone-7-O-glucoside-6″-O-malonyltransferase |
| IFH | isoflavone hydroxylase |
| IFR | isoflavone reductase |
| IFS | isoflavone synthase |
| IκB | inhibitory protein of κB family |
| IKK | IκB kinase |
| IL-1 | interleukin 1 |
| IMT | isoflavone malonyl transferase |
| iNOS | inducible nitric oxide synthases |
| INSR | insulin receptor |
| IRβ | insulin receptor beta |
| IRS1/2 | insulin receptor substrate-1 and 2 |
| ISN1 | inosine 5′-monophosphate (IMP)-specific 5′-nucleotidase |
| JAK2 | Janus kinase 2 |
| LAR | flavan-3,4-diol reductase |
| LDL | low-density lipoprotein |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| Maf | musculoaponeurotic fibrosarcoma protein |
| MAPK | mitogen-activated protein kinase |
| MEK | mitogen-activated protein kinase kinase |
| MKK1/7 | mitogen-activated protein kinase kinase 1 and 7 |
| MMP | matrix metalloproteinase |
| MT | malonyltransferase |
| MCP1 | monocyte chemoattractant protein 1 |
| MDA | malonaldehyde |
| MGO | methylglyoxal |
| NAD+ | nicotinamide adenine dinucleotide (oxidized form) |
| NADPH | nicotinamide adenine dinucleotide phosphate (reduced form) |
| NFATc1 | nuclear factor of activated T-cells cytoplasmic 1 |
| NFIL3 | nuclear factor interleukin-3-regulated protein |
| NF-κB | nuclear factor-kappa B |
| NGF | nerve growth factor |
| NIK | NF-κB-inducing kinase |
| nNOS | neuronal isoform of nitric oxide synthase |
| NO | nitrix oxide |
| NOX | NADPH oxidase |
| NOX4 | NAPDH oxidase 4 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2 |
| OCN | osteocalcin |
| OPG | osteoprotegerin |
| PAL | phenylalanine ammonia lyase |
| p-AMPK | phosphorylated adenosine 5′-monophosphate-activated protein kinase |
| PARP | poly(ADP-ribose)-polymerase |
| PDK1/2 | 3-phosphoinositide–dependent protein kinase 1 and 2 |
| PEPCK | phosphoenolpyruvate carboxykinase |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| PG | prostaglandin |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator-1alpha |
| PGE2 | prostaglandin E2 |
| PGF2 | prostaglandin F2 |
| PI3K | phosphatidylinositol 3-kinase |
| PIP2 | phosphatidylinositol-4,5-biphosphate |
| PIP3 | phosphatidylinositol-3,4,5-triphosphate |
| PKA | protein kinase A (cAMP-dependent protein kinase) |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PPARα | peroxisome proliferator-activated receptor-alpha |
| PPARγ | peroxisome proliferator-activated receptors-gamma |
| PTS | pterocarpan synthase |
| PYK | pyruvate kinase |
| RANKL | receptor activator of NF-κB ligand |
| RIP | receptor-interacting protein |
| ROS | reactive oxygen species |
| RUNX2 | runt-related transcription factor 2 |
| S1P and S2P | site-specific protease 1 and 2 |
| sAPP | soluble APP |
| SCAP | SREB cleavage-activating protein |
| SIRT1 | sirtuin 1 |
| SCD1 | stearoyl-CoA desaturase 1 |
| SGK1 | serine/threonine-protein kinase |
| SMI | structure model index |
| SOD | superoxide dismutase |
| SREB | sterol regulatory element binding |
| STAT1 | signal transducer and activator of transcription 1 |
| STEBp1 | SET-binding protein 1 |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TAK1 | transforming growth factor β-activated kinase |
| TAT1 | signal transducer and activator of transcription 1 |
| TBARs | thiobarbituric acid reactive substances |
| Tb.N | trabecular number |
| Tb.Pf | trabecular pattern factor |
| Tb.Sp | trabecular separation |
| Tb.Th | trabecular thickness |
| TCF | T cell factor |
| TG | Triacylglycerol |
| TGF-α | transforming growth factor-alpha |
| TNF | tumor necrosis factor |
| TNF-α | tumor necrosis factor-alpha |
| TNFR | tumor necrosis factor receptor |
| TNFR1 | TNF receptor-1 |
| TORC2 | target of rapamycin complex 2 |
| TRADD | TNF receptor-associated death domain |
| TRAF2 | TNF receptor-associated factor 2 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRAP | tartrate-resistant acid phosphatase |
| Ub | ubiquitination |
| UCP1 | uncoupling protein 1 |
| VR | vestitone reductase |
| Wnt/β-catenin | canonical Wnt pathway |
| XRE | xenobiotic response element |
References
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Munoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer. Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, C.R. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug. Saf. 2001, 24, 665–682. [Google Scholar] [CrossRef]
- Petrine, J.C.P.; Del Bianco-Borges, B. The influence of phytoestrogens on different physiological and pathological processes: An overview. Phytother. Res. 2021, 35, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Bacciottini, L.; Falchetti, A.; Pampaloni, B.; Bartolini, E.; Carossino, A.M.; Brandi, M.L. Phytoestrogens: Food or drug? Clin. Cases Miner. Bone Metab. 2007, 4, 123–130. [Google Scholar] [PubMed]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current perspectives on the physiological activities of fermented soybean-derived cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, C.H.; Yang, W.S. Physiologically active molecules and functional properties of soybeans in human health—A current perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.H.; Surh, J.; Kang, S.A.; Jang, K.H. Aglycone isoflavones and exopolysaccharides produced by Lactobacillus acidophilus in fermented soybean paste. Prev. Nutr. Food Sci. 2016, 21, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone supplements for menopausal women: A Systematic review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [Green Version]
- Miadokova, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferriere, F.; Pakdel, F. Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- He, F.J.; Chen, J.Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Well. 2013, 2, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Calderon, M.; Perez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Marquez, A.J. Flavonoids and isoflavonoids biosynthesis in the model legume Lotus japonicus; Connections to nitrogen metabolism and photorespiration. Plants 2020, 9, 774. [Google Scholar] [CrossRef]
- Gupta, O.P.; Nigam, D.; Dahuja, A.; Kumar, S.; Vinutha, T.; Sachdev, A.; Praveen, S. Regulation of isoflavone biosynthesis by miRNAs in two contrasting soybean genotypes at different seed developmental stages. Front. Plant Sci. 2017, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol. 2000, 124, 781–794. [Google Scholar] [CrossRef] [Green Version]
- Akashi, T.; Aoki, T.; Ayabe, S. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 2005, 137, 882–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.Z.; Li, P.; Wang, J.; Rehman, N.U.; Zhao, J. Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Front. Plant Sci. 2017, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 2017, 58, 1594–1600. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.A.R.; Watanabe, S.; Suzuki, A.; Hashimoto, F.; Anai, T. Identification of novel MYB transcription factors involved in the isoflavone biosynthetic pathway by using the combination screening system with agroinfiltration and hairy root transformation. Plant Biotechnol. 2019, 36, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.E.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015, 6, 2017–2025. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Jayaraman, D.; Forshey, K.L.; Grimsrud, P.A.; Ane, J.M. Leveraging proteomics to understand plant-microbe interactions. Front. Plant Sci. 2012, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, A.; McDowell, T.; Chen, L.; Renaud, J.; Dhaubhadel, S. Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 2018, 96, 966–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, I.; Salvatori, C.; Di Cara, G.; Latini, A.; Frati, F.; Troiani, S.; Principi, N.; Esposito, S. Soy-based infant formula: Are phyto-oestrogens still in doubt? Front. Nutr. 2018, 5, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef] [Green Version]
- Skalicky, M.; Kubes, J.; Hejnak, V.; Tumova, L.; Martinkova, J.; Martin, J.; Hnilickova, H. Isoflavones production and possible mechanism of their exudation in Genista tinctoria L. suspension culture after treatment with vanadium compounds. Molecules 2018, 23, 1619. [Google Scholar] [CrossRef] [Green Version]
- Kubes, J.; Skalicky, M.; Tumova, L.; Martin, J.; Hejnak, V.; Martinkova, J. Vanadium elicitation of Trifolium pratense L. cell culture and possible pathways of produced isoflavones transport across the plasma membrane. Plant Cell Rep. 2019, 38, 657–671. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef]
- Romera, F.J.; Garcia, M.J.; Lucena, C.; Martinez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcantara, E.; Angulo, M.; Perez-Vicente, R. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Conrath, U. Systemic acquired resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Toth, K.; Stacey, G. Does plant immunity play a critical role during initiation of the legume-rhizobium symbiosis? Front. Plant Sci. 2015, 6, 401. [Google Scholar] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef]
- Lygin, A.V.; Zernova, O.V.; Hill, C.B.; Kholina, N.A.; Widholm, J.M.; Hartman, G.L.; Lozovaya, V.V. Glyceollin is an important component of soybean plant defense against Phytophthora sojae and Macrophomina phaseolina. Phytopathology 2013, 103, 984–994. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoids and isoflavones: Absorption, metabolism, and bioactivity. Free Radic. Biol. Med. 2004, 36, 827–828. [Google Scholar] [CrossRef]
- Setchell, K.D. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. J. Nutr. 2000, 130, 654S–655S. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Maeda, H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules 2017, 23, 74. [Google Scholar] [CrossRef] [Green Version]
- Huser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Kohrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef] [Green Version]
- Simons, R.; Gruppen, H.; Bovee, T.F.; Verbruggen, M.A.; Vincken, J.P. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs). Food Funct. 2012, 3, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Toloi, M.R.T.; Vasconcelos, L.D.; Fonseca, M.J.V.; Correia-da-Silva, G.; Teixeira, N. The role of soybean extracts and isoflavones in hormone-dependent breast cancer: Aromatase activity and biological effects. Food Funct. 2017, 8, 3064–3074. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Wang, D.; Zhang, L.; Lv, J.; Jiang, N.; Fan, B.; Liu, X.; Wang, F. Neuroprotective effects of soy isoflavones on scopolamine-induced amnesia in mice. Nutrients 2018, 10, 853. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; An, Y.; Lv, C.; Ma, W.; Xi, Y.; Xiao, R. Dietary soybean isoflavones in Alzheimer’s disease prevention. Asia Pac. J. Clin. Nutr. 2018, 27, 946–954. [Google Scholar]
- Uddin, M.S.; Kabir, M.T. Emerging signal regulating potential of genistein against Alzheimer’s disease: A promising molecule of interest. Front. Cell Dev. Biol. 2019, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Garrido, P.; Cabello, E.; Alonso, A.; Gonzalez, C. Effects of estradiol and genistein on the insulin signaling pathway in the cerebral cortex of aged female rats. Exp. Gerontol. 2014, 58, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Jin, G.; Zhao, M.; Yang, H. The effect of genistein on the content and activity of alpha- and beta-secretase and protein kinase C in Abeta-injured hippocampal neurons. Basic Clin. Pharmacol. Toxicol. 2013, 112, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, X.Q.; Ding, C.; Du, X.L. Genistein attenuates isoflurane-induced neurotoxicity and improves impaired spatial learning and memory by regulating cAMP/CREB and BDNF-TrkB-PI3K/Akt signaling. Korean J. Physiol. Pharmacol. 2017, 21, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.H.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. 6,7,4’-Trihydroxyisoflavone, a major metabolite of daidzein, improves learning and memory via the cholinergic system and the p-CREB/BDNF signaling pathway in mice. Eur. J. Pharmacol. 2018, 826, 140–147. [Google Scholar] [CrossRef]
- Rizzo, G. The antioxidant role of soy and soy foods in human health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Gleason, C. Evaluation of the potential antidepressant effects of soybean isoflavones. Menopause 2016, 23, 1348–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.A.; Cuthbert, B.; Lewis-Fernandez, R.; Narrow, W.E.; Reed, G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest. 2017, 18, 72–145. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Ma, L.; Wang, Y.G.; Ye, F.; Wang, C.; Zhou, W.H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Soy isoflavone intake and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. Eur. J. Nutr. 2018, 57, 441–450. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Pischon, T.; Nothlings, U.; Boeing, H. Obesity and cancer. Proc. Nutr. Soc. 2008, 67, 128–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, S.; Fujita, M.; Kamei, Y. Health promotion effects of soy isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Pan, M.H.; Ho, C.T. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhao, A.; Wu, Y.; Zhao, Y.; Yang, X. Soybean soluble polysaccharides enhance bioavailability of genistein and its prevention against obesity and metabolic syndrome of mice with chronic high fat consumption. Food Funct. 2019, 10, 4153–4165. [Google Scholar] [CrossRef]
- Grossini, E.; Farruggio, S.; Raina, G.; Mary, D.; Deiro, G.; Gentilli, S. Effects of genistein on differentiation and viability of human visceral adipocytes. Nutrients 2018, 10, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, J.J.; Lee, D.E.; Lee, C.H.; Shin, C.S. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. J. Food Sci. Technol. 2016, 53, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017, 61, 1600574. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Pang, D.; Luo, Q.; Chen, X.; Gao, Q.; Shi, L.; Liu, W.; Zou, Y.; Li, L.; Chen, Z. Soy isoflavones regulate lipid metabolism through an AKT/mTORC1 pathway in diet-induced obesity (DIO) male rats. Molecules 2016, 21, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Kurrat, A.; Blei, T.; Kluxen, F.M.; Mueller, D.R.; Piechotta, M.; Soukup, S.T.; Kulling, S.E.; Diel, P. Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol. Nutr. Food Res. 2015, 59, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. 2005, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagawa, N.; Kubota, S.; Kobayashi, Y.; Kato, I. Genistein inhibits glucocorticoid amplification in adipose tissue by suppression of 11beta-hydroxysteroid dehydrogenase type 1. Steroids 2015, 93, 77–86. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Cakala-Jakimowicz, M.; Puzianowska-Kuznicka, M. Targeting abdominal obesity and its complications with dietary phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef] [Green Version]
- Kurylowicz, A. The role of isoflavones in type 2 diabetes prevention and treatment-A narrative review. Int. J. Mol. Sci. 2020, 22, 218. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.A.; Mahmoud, M.M.A.; Tag, H.M.; Greish, S.M.; Eltamany, D.A.; Soliman, M.T.A. Soy isoflavones ameliorate metabolic and immunological alterations of ovariectomy in female Wistar rats: Antioxidant and estrogen sparing potential. Oxid. Med. Cell Longev. 2019, 2019, 5713606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotundo, L.; Persaud, A.; Feurdean, M.; Ahlawat, S.; Kim, H.S. The association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin. Mol. Hepatol. 2018, 24, 392–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Loste, M.T.; Ranchal, I.; Romero-Gomez, M.; Crespo, J. Irisin, a link among fatty liver disease, physical inactivity and insulin resistance. Int. J. Mol. Sci. 2014, 15, 23163–23178. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Una, M.; Lopez-Mancheno, Y.; Dieguez, C.; Fernandez-Rojo, M.A.; Novelle, M.G. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Hemati, N.; Asis, M.; Moradi, S.; Mollica, A.; Stefanucci, A.; Nikfar, S.; Mohammadi, E.; Farzaei, M.H.; Abdollahi, M. Effects of genistein on blood pressure: A systematic review and meta-analysis. Food Res. Int. 2020, 128, 108764. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Shin, H.; Song, J.H.; Kwak, S.H.; Kang, S.M.; Yoon, J.W.; Choi, S.H.; Cho, S.I.; Park, K.S.; Lee, H.K.; et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011, 34, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Soy isoflavones and cholecalciferol reduce inflammation, and gut permeability, without any effect on antioxidant capacity in irritable bowel syndrome: A randomized clinical trial. Clin. Nutr. ESPEN 2019, 34, 50–54. [Google Scholar] [CrossRef]
- Abron, J.D.; Singh, N.P.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS ONE 2018, 13, e0199631. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, R.; Zhang, X.; Wang, Z.; Wen, J.; Zhou, T. Non-isoflavones diet incurred metabolic modifications induced by constipation in rats via targeting gut microbiota. Front. Microbiol. 2018, 9, 3002. [Google Scholar] [CrossRef]
- Paley, C.A.; Johnson, M.I. Abdominal obesity and metabolic syndrome: Exercise as medicine? BMC Sports Sci. Med. Rehabil. 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H. The vascular effects of isolated isoflavones—A focus on the determinants of blood pressure regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar]
- Harsha, D.W.; Bray, G.A. Weight loss and blood pressure control (Pro). Hypertension 2008, 51, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.W.; Chen, N.W.; Nayeem, F.; Nagamani, M.; Anderson, K.E. Soy isoflavones interact with calcium and contribute to blood pressure homeostasis in women: A randomized, double-blind, placebo controlled trial. Eur. J. Nutr. 2020, 59, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Chen, C.M.; Hsu, C.Y. Effect of home telehealth care on blood pressure control: A public healthcare centre model. J. Telemed. Telecare 2019, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.I.; Steffen, L.M.; Swett, K.; Smith, C.; Burke, L.; Zhou, X.; Shikany, J.M.; Rodriguez, C.J. Dietary total isoflavone intake is associated with lower systolic blood pressure: The coronary artery risk development in young adults (CARDIA) study. J. Clin. Hypertens. 2016, 18, 778–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.R.; Chen, K.H. Utilization of isoflavones in soybeans for women with menopausal syndrome: An overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choue, R.; Lim, H. Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: A randomized clinical trial. Nutr. Res. Pract. 2017, 11, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, J.; Park, K. Association between soy food and dietary soy isoflavone intake and the risk of cardiovascular disease in women: A prospective cohort study in Korea. Nutrients 2021, 13, 1407. [Google Scholar] [CrossRef] [PubMed]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The potential effects of phytoestrogens: The role in neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Qin, W.; Du, N.; Zhang, L.; Wu, X.; Hu, Y.; Li, X.; Shen, N.; Li, Y.; Yang, B.; Xu, C.; et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br. J. Pharmacol. 2015, 172, 5559–5572. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Leu, S.Y.; Peng, Y.J.; Lee, Y.M.; Hsu, C.H.; Chou, S.C.; Yen, M.H.; Cheng, P.Y. Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation. J. Cell. Mol. Med. 2017, 21, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Shu, F.; Zeng, Y.; Meng, X.; Wang, B.; Diao, L.; Wang, L.; Wan, J.; Zhu, J.; Wang, J.; et al. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-beta RsaI. J. Nutr. 2014, 144, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Preston, R.A. Effects of blood pressure reduction on cardiovascular risk estimates in hypertensive postmenopausal women. Climacteric 2007, 10, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.W.; Taylor, A.A.; Smith, E.O.; Barnes, S.; Hachey, D.L. Effect of soy isoflavone supplementation on nitric oxide metabolism and blood pressure in menopausal women. Am. J. Clin. Nutr. 2012, 95, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Kim, D.J. Epidemiology of alcoholic liver disease in Korea. Clin. Mol. Hepatol. 2018, 24, 93–99. [Google Scholar] [CrossRef]
- Xiao, C.W.; Wood, C.M.; Weber, D.; Aziz, S.A.; Mehta, R.; Griffin, P.; Cockell, K.A. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr. 2014, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015, 7, 1450–1459. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, H.; Leng, L.; Jiang, Z. Effects of soy isoflavone on hepatic steatosis in high fat-induced rats. J. Clin. Biochem. Nutr. 2017, 61, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Liu, J.; Wang, K.; Guo, X.; Ji, B.; Wu, W.; Zhou, F. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agric. Food Chem. 2016, 64, 7291–7297. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.Y.; Jeon, S.; Nam, Y.; Park, Y.J.; Won, S.B.; Kwon, Y.H. Dietary supplementation of genistein alleviates liver inflammation and fibrosis mediated by a methionine-choline-deficient diet in db/db mice. J. Agric. Food Chem. 2015, 63, 4305–4311. [Google Scholar] [CrossRef]
- Jing, Z.; Wei-Jie, Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2016, 35, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ranich, T.; Bhathena, S.J.; Velasquez, M.T. Protective effects of dietary phytoestrogens in chronic renal disease. J. Ren. Nutr. 2001, 11, 183–193. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Dietary phytoestrogens: A possible role in renal disease protection. Am. J. Kidney Dis. 2001, 37, 1056–1068. [Google Scholar] [CrossRef]
- Li, W.F.; Yang, K.; Zhu, P.; Zhao, H.Q.; Song, Y.H.; Liu, K.C.; Huang, W.F. Genistein ameliorates ischemia/reperfusion-induced renal injury in a SIRT1-dependent manner. Nutrients 2017, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Tang, N.; Woo, J. Effect of whole soy and purified isoflavone daidzein on renal function—A 6-month randomized controlled trial in equol-producing postmenopausal women with prehypertension. Clin. Biochem. 2014, 47, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.F.; Hayashi, K.; Matsumura, Y.; Kawada, T.; Seno, S.; Matsuda, H.; Inoue, K.; Nomura, W.; Takahashi, H.; Goto, T. Anti-inflammatory and antioxidative properties of isoflavones provide renal protective effects distinct from those of dietary soy proteins against diabetic nephropathy. Mol. Nutr. Food Res. 2020, 64, e2000015. [Google Scholar] [CrossRef]
- Liu, C.L.; Yan, L.; Cai, K.R.; Sun, K.; Qi, Y.; Han, Y.L.; Zhang, X.D.; Sun, X.D. Effects of soybean isoflavones on Wnt/beta-catenin and the TGF-beta1 signaling pathway in renal tissue of type 2 diabetic rats. J. Biol. Regul. Homeost. Agents 2018, 32, 455–464. [Google Scholar] [PubMed]
- Kim, M.J.; Lim, Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediators Inflamm. 2013, 2013, 510212. [Google Scholar] [CrossRef] [Green Version]
- Schreihofer, D.A.; Oppong-Gyebi, A. Genistein: Mechanisms of action for a pleiotropic neuroprotective agent in stroke. Nutr. Neurosci. 2019, 22, 375–391. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Wei, A.; Bi, F.; Zhu, X.; Yin, S.; Lin, W.; Cao, W. Klotho recovery by genistein via promoter histone acetylation and DNA demethylation mitigates renal fibrosis in mice. J. Mol. Med. 2019, 97, 541–552. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [Green Version]
- Bernatoniene, J.; Kazlauskaite, J.A.; Kopustinskiene, D.M. Pleiotropic effects of isoflavones in inflammation and chronic degenerative diseases. Int. J. Mol. Sci. 2021, 22, 5656. [Google Scholar] [CrossRef]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of intestinal inflammation by soybean and soy-derived compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Ghasemi, A.; Baradaran, H.R.; Mollanoroozy, E.; Gholami, A. Beneficial effect of soy isoflavones and soy isoflavones plus soy protein on serum concentration of C-reactive protein among postmenopausal women: An updated systematic review and meta-analysis of randomized controlled trials. Complement Ther. Med. 2021, 59, 102715. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Abernathy, L.M.; Fountain, M.D.; Rothstein, S.E.; David, J.M.; Yunker, C.K.; Rakowski, J.; Lonardo, F.; Joiner, M.C.; Hillman, G.G. Soy isoflavones promote radioprotection of normal lung tissue by inhibition of radiation-induced activation of macrophages and neutrophils. J. Thorac. Oncol. 2015, 10, 1703–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abernathy, L.M.; Fountain, M.D.; Joiner, M.C.; Hillman, G.G. Innate immune pathways associated with lung radioprotection by soy isoflavones. Front. Oncol. 2017, 7, 7. [Google Scholar] [CrossRef]
- Feng, G.; Sun, B.; Li, T.Z. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. Int. Immunopharmacol. 2015, 26, 392–400. [Google Scholar] [CrossRef]
- Liu, X.J.; Bao, H.R.; Zeng, X.L.; Wei, J.M. Effects of resveratrol and genistein on nuclear factor kappaB, tumor necrosis factor alpha and matrix metalloproteinase 9 in patients with chronic obstructive pulmonary disease. Mol. Med. Rep. 2016, 13, 4266–4272. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.; Singh, T.U.; Thangamalai, R.; Narasimha Reddy, C.E.; Panigrahi, M.; Kandasamy, K.; Singh, V.; Mishra, S.K. Daidzein pretreatment improves survival in mouse model of sepsis. J. Surg. Res. 2015, 197, 363–373. [Google Scholar] [CrossRef]
- Prasad, K. Homocysteine, a risk factor for cardiovascular disease. Int. J. Angiol. 1999, 8, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef]
- Susutlertpanya, W.; Werawatganon, D.; Siriviriyakul, P.; Klaikeaw, N. Genistein attenuates nonalcoholic steatohepatitis and increases hepatic PPARγ in a rat model. Evid. Based. Complement. Alternat. Med. 2015, 2015, 509057. [Google Scholar] [CrossRef] [Green Version]
- Ganai, A.A.; Khan, A.A.; Malik, Z.A.; Farooqi, H. Genistein modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicol. Appl. Pharmacol. 2015, 283, 139–146. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp. Ther. Med. 2017, 14, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kong, D.; Bao, B.; Ahmad, A.; Sarkar, F.H. Induction of cancer cell death by isoflavone: The role of multiple signaling pathways. Nutrients 2011, 3, 877–896. [Google Scholar] [CrossRef] [Green Version]
- Diana, T.; Brown, R.S.; Bossowski, A.; Segni, M.; Niedziela, M.; Konig, J.; Bossowska, A.; Ziora, K.; Hale, A.; Smith, J.; et al. Clinical relevance of thyroid-stimulating autoantibodies in pediatric graves’ disease-a multicenter study. J. Clin. Endocrinol. Metab. 2014, 99, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.N.; Cotterchio, M.; Boucher, B.A.; Kreiger, N. Phytoestrogen intake from foods, during adolescence and adulthood, and risk of breast cancer by estrogen and progesterone receptor tumor subgroup among Ontario women. Int. J. Cancer. 2013, 132, 1683–1692. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy consumption and the risk of prostate cancer: An updated systematic review and meta-analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.C.; Wong, E.L.; Li, H.; You, J.H.; Ho, S.; Woo, J.; Hui, E. Isoflavones in treating watchful waiting benign prostate hyperplasia: A double-blinded, randomized controlled trial. J. Altern. Complement. Med. 2012, 18, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.P.; Chien, M.H. Phytoestrogens induce apoptosis through a mitochondria/caspase pathway in human breast cancer cells. Climacteric 2014, 17, 385–392. [Google Scholar] [CrossRef]
- Zhang, X.; Cook, K.L.; Warri, A.; Cruz, I.M.; Rosim, M.; Riskin, J.; Helferich, W.; Doerge, D.; Clarke, R.; Hilakivi-Clarke, L. Lifetime genistein intake increases the response of mammary tumors to Tamoxifen in tats. Clin. Cancer Res. 2017, 23, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Shin, H.S.; Lee, Y.S.; Lee, D.; Kim, S.; Lee, Y.C. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncol. Rep. 2014, 31, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Q.; Wang, X.; Wang, X.; Huang, Z.; Jiao, Y.; Wang, J. Quantitiative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int. J. Oncol. 2016, 48, 1016–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Kim, H.S.; Song, Y.S. Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Roh, T.; Kim, S.W.; Moon, S.H.; Nam, M.J. Genistein induces apoptosis by down-regulating thioredoxin-1 in human hepatocellular carcinoma SNU-449 cells. Food Chem. Toxicol. 2016, 97, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Zang, A.; Jia, Y.; Shang, Y.; Zhang, Z.; Ge, K.; Zhang, J.; Fan, W.; Wang, B. Genistein inhibits A549 human lung cell proliferation via miR-27a and MET signaling. Oncol. Lett. 2016, 12, 2189–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Li, J.; Li, B.; Wang, Y.; Li, M.; Ma, D.; Wang, X. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumour Biol. 2014, 35, 4137–4145. [Google Scholar] [CrossRef] [PubMed]
- Jaudan, A.; Sharma, S.; Malek, S.N.A.; Dixit, A. Induction of apoptosis by pinostrobin in human cervical cancer cells: Possible mechanism of action. PLoS ONE 2018, 13, e0191523. [Google Scholar] [CrossRef] [Green Version]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural products targeting ER stress, and the functional link to mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranche, S.; Brotons, C.; Pascual de la Pisa, B.; Macias, R.; Hevia, E.; Marzo-Castillejo, M. Impact of a soy drink on climacteric symptoms: An open-label, crossover, randomized clinical trial. Gynecol. Endocrinol. 2016, 32, 477–482. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Razeghi, S.; Gorji, A.; Tabaraei, A.; Wada, K.; Tsuji, M.; Tamura, T.; Konishi, K.; Kawachi, T.; et al. Soy isoflavone intake and stomach cancer risk in Japan: From the Takayama study. Int. J. Cancer 2015, 137, 885–892. [Google Scholar]
- Moradi, A.; Alizadeh, A.; Vakili, M.A. Genistein induces a protective immunomodulatory effect in a mouse model of cervical cancer. Iran J. Immunol. 2012, 9, 119–127. [Google Scholar]
- Liu, Z.M.; Ho, S.; Hao, Y.T.; Chen, Y.M.; Woo, J.; Wong, S.Y.; He, Q.; Xie, Y.J.; Tse, L.A.; Chen, B.; et al. Randomised controlled trial of effect of whole soy replacement diet on features of metabolic syndrome in postmenopausal women: Study protocol. BMJ Open 2016, 6, e012741. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Jing, X.; Li, H.; Zhao, X.; Wang, D. Soy isoflavone consumption and colorectal cancer risk: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 25939. [Google Scholar] [CrossRef]
- Shin, A.; Lee, J.; Lee, J.; Park, M.S.; Park, J.W.; Park, S.C.; Oh, J.H.; Kim, J. Isoflavone and soyfood intake and colorectal cancer risk: A case-control study in Korea. PLoS ONE 2015, 10, e0143228. [Google Scholar]
- Lee, A.H.; Su, D.; Pasalich, M.; Tang, L.; Binns, C.W.; Qiu, L. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr. Res. 2014, 34, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Choi, K.C.; Hwang, K.A. Genistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-β signaling pathway. Phytomedicine 2015, 22, 993–999. [Google Scholar] [CrossRef]
- Lazarevic, B.; Hammarstrom, C.; Yang, J.; Ramberg, H.; Diep, L.M.; Karlsen, S.J.; Kucuk, O.; Saatcioglu, F.; Tasken, K.A.; Svindland, A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br. J. Nutr. 2012, 108, 2138–2147. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Bilir, B.; Sharma, N.V.; Lee, J.; Hammarstrom, B.; Svindland, A.; Kucuk, O.; Moreno, C.S. Effects of genistein supplementation on genomewide DNA methylation and gene expression in patients with localized prostate cancer. Int. J. Oncol. 2017, 51, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Mayo, B.; Vazquez, L.; Florez, A.B. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-equol, a secondary metabolite of natural anticancer isoflavone daidzein, inhibits prostate cancer growth in vitro and in vivo, though activating the Akt/FOXO3a pathway. Curr. Cancer Drug Targets 2016, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lesinski, G.B.; Reville, P.K.; Mace, T.A.; Young, G.S.; Ahn-Jarvis, J.; Thomas-Ahner, J.; Vodovotz, Y.; Ameen, Z.; Grainger, E.; Riedl, K.; et al. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev. Res. 2015, 8, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhang, L.; Na, R.; Xu, J.; Xiong, Z.; Zhang, N.; Dai, W.; Jiang, H.; Ding, Q. Plasma genistein and risk of prostate cancer in Chinese population. Int. Urol. Nephrol. 2015, 47, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, E.; Zhang, T.; Sun, X.; Li, Y.; Geng, H.; Yu, D.; Zhong, C. Sonic hedgehog pathway mediates genistein inhibition of renal cancer stem cells. Oncol. Lett. 2019, 18, 3081–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, H.; Zhao, Z.; Luo, M.; Ju, Y.; Yang, G.; Mei, Z. Genistein suppresses allergic contact dermatitis through regulating the MAP2K2/ERK pathway. Food Funct. 2021, 12, 4556–4569. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Bustamante, F.A.; Bhoola, K.D.; Figueroa, C.D.; Ehrenfeld, P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci. 2018, 132, 2583–2598. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Impact of soy foods on the development of breast cancer and the prognosis of breast cancer patients. Forsch. Komplementmed. 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Betancourt, A.M.; Wang, J.; Jenkins, S.; Mobley, J.; Russo, J.; Lamartiniere, C.A. Altered carcinogenesis and proteome in mammary glands of rats after prepubertal exposures to the hormonally active chemicals bisphenol A and genistein. J. Nutr. 2012, 142, 1382S–1388S. [Google Scholar] [CrossRef] [Green Version]
- Van Duursen, M.B.; Nijmeijer, S.M.; de Morree, E.S.; de Jong, P.C.; van den Berg, M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 2011, 289, 67–73. [Google Scholar] [CrossRef]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Goeppinger, T.S.; Doerge, D.R.; Helferich, W.G. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis 2004, 25, 1649–1657. [Google Scholar] [CrossRef]
- Allred, C.D.; Ju, Y.H.; Allred, K.F.; Chang, J.; Helferich, W.G. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis 2001, 22, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Poschner, S.; Maier-Salamon, A.; Zehl, M.; Wackerlig, J.; Dobusch, D.; Pachmann, B.; Sterlini, K.L.; Jager, W. The impacts of genistein and daidzein on estrogen conjugations in human breast cancer cells: A targeted metabolomics approach. Front. Pharmacol. 2017, 8, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurkiewicz-Przondziono, J.; Lemm, M.; Kwiatkowska-Pamula, A.; Ziolko, E.; Wojtowicz, M.K. Influence of diet on the risk of developing endometriosis. Ginekol. Pol. 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Mvondo, M.A.; Ekenfack, J.D.; Minko Essono, S.; Saah Namekong, H.; Awounfack, C.F.; Laschke, M.W.; Njamen, D. Soy intake since the prepubertal age may contribute to the pathogenesis of endometriosis in adulthood. J. Med. Food 2019, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.C.; Anaf, V.; Fayt, I.; Wespes, E. Ureteral mullerian carcinosarcoma (mixed mullerian tumor) associated with endometriosis occurring in a patient with a concentrated soy isoflavones supplementation. Arch. Gynecol. Obstet. 2006, 274, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Sea, J.L.; Abramyan, M.; Chiu, H.K. Prepubescent unilateral gynecomastia secondary to excessive soy consumption. J. Pediatr. Endocrinol. Metab. 2021, 34, 521–525. [Google Scholar] [CrossRef]
- Martinez, J.; Lewi, J.E. An unusual case of gynecomastia associated with soy product consumption. Endocr. Pract. 2008, 14, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziaei, S.; Halaby, R. Dietary isoflavones and breast cancer risk. Medicines 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, O. Soy foods, isoflavones, and breast cancer. Cancer 2017, 123, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- Kanadys, W.; Baranska, A.; Blaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Malm, M.; Kanecki, K. Effects of soy isoflavones on biochemical markers of bone metabolism in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2021, 18, 5346. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender disparities in osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic. Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar]
- Ho, S.C.; Wong, E.; Chan, S.G.; Lau, J.; Chan, C.; Leung, P.C. Determinants of peak bone mass in Chinese women aged 21-40 years. III. Physical activity and bone mineral density. J. Bone Miner. Res. 1997, 12, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The determinants of peak bone mass. J. Pediatr. 2017, 180, 261–269. [Google Scholar] [CrossRef]
- Noh, D.; Lim, Y.; Lee, H.; Kim, H.; Kwon, O. Soybean-Hop alleviates estrogen deficiency-related bone loss and metabolic dysfunction in ovariectomized rats fed a high-fat diet. Molecules 2018, 23, 1205. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Liu, Z.; Tong, Z.; Zhao, Z.; Liang, H. Soybean isoflavone treatment induces osteoblast differentiation and proliferation by regulating analysis of Wnt/beta-catenin pathway. Gene 2015, 573, 273–277. [Google Scholar] [CrossRef]
- Ahn, H.; Park, Y.K. Soy isoflavone supplementation improves longitudinal bone growth and bone quality in growing female rats. Nutrition 2017, 37, 68–73. [Google Scholar] [CrossRef]
- Santos, M.A.; Florencio-Silva, R.; Medeiros, V.P.; Nader, H.B.; Nonaka, K.O.; Sasso, G.R.; Simoes, M.J.; Reginato, R.D. Effects of different doses of soy isoflavones on bone tissue of ovariectomized rats. Climacteric 2014, 17, 393–401. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy Isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Ward, W.E.; Kaludjerovic, J.; Dinsdale, E.C. A mouse model for studying nutritional programming: Effects of early life exposure to soy isoflavones on bone and reproductive health. Int. J. Environ. Res. Public Health 2016, 13, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Lee, H.S.; Jung, J.I.; Lim, S.M.; Lim, J.H.; Ha, W.H.; Jeon, C.L.; Lee, J.Y.; Kim, E.J. Effect of isoflavone-enriched whole soy milk powder supplementation on bone metabolism in ovariectomized mice. Nutr. Res. Pract. 2018, 12, 275–282. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef] [PubMed]
- Maliehe, A.; Ghahremani, S.; Kharghani, S.; Ghazanfarpour, M.; Shariati, K.; Kazemi, M.; Khadivzadeh, T. Effect of isoflavones and genistein on glucose metabolism in peri- and post-menopausal women: An overview of meta-analysis. J. Menopausal Med. 2019, 25, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, J.; Shi, J. Management of diabetes mellitus with puerarin, a natural isoflavone from Pueraria lobata. Am. J. Chin. Med. 2018, 46, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; Bramer, W.M.; Ahmadizar, F.; Chowdhury, R.; Danser, A.J.; Roks, A.J.; Voortman, T.; Franco, O.H.; et al. Associations between phytoestrogens, glucose homeostasis, and risk of diabetes in women: A systematic review and meta-analysis. Adv. Nutr. 2018, 9, 726–740. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shaw, N.S.; Tsai, K.S.; Chen, C.Y. The hypoglycemic effects of soy isoflavones on postmenopausal women. J. Womens Health 2004, 13, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Liu, D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.B.; Chen, W.H.; Guo, J.J.; Fu, Z.H.; Yi, C.; Zhang, M.; Na, X.L. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women—A meta-analysis. Nutrition 2013, 29, 8–14. [Google Scholar] [CrossRef]
- Jamilian, M.; Asemi, Z. The effects of soy isoflavones on metabolic status of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3386–3394. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.L.; Germolec, D.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.; Mittal, A.; Dabur, R. Mechanistic approach of anti-diabetic compounds identified from natural sources. Chem. Biol. Lett. 2018, 5, 63–99. [Google Scholar]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Antihyperglycemic effect of equol, a daidzein derivative, in cultured L6 myocytes and ob/ob mice. Mol. Nutr. Food Res. 2014, 58, 267–277. [Google Scholar] [CrossRef]
- Li, G.; Prior, J.C.; Leslie, W.D.; Thabane, L.; Papaioannou, A.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Anastassiades, T.; Towheed, T.; et al. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care 2019, 42, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Fraser, W.D.; Kilpatrick, E.S.; Atkin, S.L. Effect of soy on bone turn-over markers in men with type 2 diabetes and hypogonadism—A randomised controlled study. Sci. Rep. 2017, 7, 15366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Franke, A.A.; Rosner, B.A.; Giovannucci, E.; van Dam, R.M.; Tworoger, S.S.; Hu, F.B.; Sun, Q. Urinary isoflavonoids and risk of type 2 diabetes: A prospective investigation in US women. Br. J. Nutr. 2015, 114, 1694–1701. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Pan, A.; Manson, J.E.; Willett, W.C.; Malik, V.; Rosner, B.; Giovannucci, E.; Hu, F.B.; Sun, Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: A pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016, 70, 1381–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Shen, M.H.; Jin, M.H.; Jin, A.H.; Yin, X.Z.; Quan, J.S. Hypoglycemic property of soy isoflavones from hypocotyl in Goto-Kakizaki diabetic rats. J. Clin. Biochem. Nutr. 2018, 62, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gao, F.; Luo, H.; Zhang, C.T.; Zhang, R. Differential response in levels of high-density lipoprotein cholesterol to one-year metformin treatment in prediabetic patients by race/ethnicity. Cardiovasc. Diabetol. 2015, 14, 79. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-equol activates cAMP signaling at the plasma membrane of INS-1 pancreatic β-cells and protects against streptozotocin-induced hyperglycemia by increasing β-Cell function in male mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Zebrowitz, L.A. First impressions from faces. Curr. Dir. Psychol. Sci. 2017, 26, 237–242. [Google Scholar] [CrossRef]
- Bochenska, K.; Gabig-Ciminska, M. Unbalanced sphingolipid metabolism and its implications for the pathogenesis of psoriasis. Molecules 2020, 25, 1130. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.Y.; Lee, G. Potential therapeutic applications of bee venom on skin disease and its mechanisms: A literature review. Toxins 2019, 11, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Do, M.H.; Oh, Y.L.; Cho, D.W.; Kim, S.H.; Kim, S.Y. Dietary fermented soybean suppresses UVB-induced skin inflammation in hairless mice via regulation of the MAPK signaling pathway. J. Agric. Food Chem. 2014, 62, 8962–8972. [Google Scholar] [CrossRef] [PubMed]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Giannini, V.; Monfrecola, G.; Bevilacqua, M.A. A comparative analysis of the photo-protective effects of soy isoflavones in their aglycone and glucoside forms. Int. J. Mol. Sci. 2012, 13, 16444–16456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z.D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, S.M.; Jung, I.K.; Lim, Y.; Kim, J.H. Effects of genistein on early-stage cutaneous wound healing. Biochem. Biophys. Res. Commun. 2011, 410, 514–519. [Google Scholar] [CrossRef]
- Kim, Y.M.; Huh, J.S.; Lim, Y.; Cho, M. Soy isoflavone glycitin (4′-hydroxy-6-methoxyisoflavone-7-D-glucoside) promotes human dermal fibroblast cell proliferation and migration via TGF-β signaling. Phytother. Res. 2015, 29, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.G.; Kim, J.E.; Lee, S.Y.; Park, J.S.; Yeom, M.H.; Chen, H.; Bode, A.M.; Dong, Z.; Lee, K.W. The daidzein metabolite, 6,7,4′-trihydroxyisoflavone, is a novel inhibitor of PKCα in suppressing solar UV-induced matrix metalloproteinase 1. Int. J. Mol. Sci. 2014, 15, 21419–21432. [Google Scholar] [CrossRef]
- Mason, A.; Kinugasa, T. East Asian economic development: Two demographic dividends. J. Asian Econ. 2008, 19, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Lee, J.A.; Son, D.; Park, D.; Jung, E. Anti-skin-aging activity of a standardized extract from Panax ginseng leaves in vitro and in human volunteer. Cosmetics 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, G.; Wainwright, L.J.; Holland, R.; Barrett, K.E.; Casey, J. Wrinkle reduction in post-menopausal women consuming a novel oral supplement: A double-blind placebo-controlled randomized study. Int. J. Cosmet. Sci. 2014, 36, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Faber, L.; Kovac, I.; Mitrengova, P.; Novotny, M.; Varinska, L.; Vasilenko, T.; Kello, M.; Coma, M.; Kuruc, T.; Petrova, K.; et al. Genistein improves skin flap viability in rats: A preliminary in vivo and in vitro investigation. Molecules 2018, 23, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.J.; Wu, N.L.; Lee, G.A.; Hung, C.F. The therapeutic potential and molecular mechanism of isoflavone extract against psoriasis. Sci. Rep. 2018, 8, 6335. [Google Scholar] [CrossRef] [Green Version]
- Back, P.I.; Furtado, L.R.; Nemitz, M.C.; Balestrin, L.A.; Fachel, F.N.S.; Gomes, H.M.; Schuh, R.S.; Moreira, J.C.; von Poser, G.L.; Teixeira, H.F. Skin permeation and oxidative protection effect of soybean isoflavones from topical nanoemulsions-a comparative study of extracts and pure compounds. AAPS PharmSciTech 2018, 19, 3029–3039. [Google Scholar] [CrossRef]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-oxidative effects of 17 β-estradiol and genistein in human skin fibroblasts and keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchnik, E.; Kruk, J.; Baranowska-Bosiacka, I.; Pilutin, A.; Maleszka, R.; Marchlewicz, M. Effects of the soy isoflavones, genistein and daidzein, on male rats’ skin. Postepy Dermatol. Alergol. 2019, 36, 760–766. [Google Scholar] [CrossRef]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef]
- Sansai, K.; Na Takuathung, M.; Khatsri, R.; Teekachunhatean, S.; Hanprasertpong, N.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2020, 31, 1853–1864. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, Y.; Jiang, C. Isoflavone combined with exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. J. Chin. Med. Assoc. 2020, 83, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Sankar, P.; Zachariah, B.; Anissa, A.M. Soy isoflavones (from Glycine max) in menopause health and diseases. Biochem.Physiol. 2017, 6, 225. [Google Scholar]
- Chilibeck, P.D.; Vatanparast, H.; Pierson, R.; Case, A.; Olatunbosun, O.; Whiting, S.J.; Beck, T.J.; Pahwa, P.; Biem, H.J. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: A randomized clinical trial. J. Bone Miner. Res. 2013, 28, 780–793. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: A 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, S.; Tong, H.; Shi, S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother. Res. 2018, 32, 384–394. [Google Scholar] [CrossRef]
- Ahsan, M.; Mallick, A.K. The effect of soy isoflavones on the menopause rating scale scoring in perimenopausal and postmenopausal women: A pilot study. J. Clin. Diagn. Res. 2017, 11, FC13–FC16. [Google Scholar] [CrossRef]
- De Franciscis, P.; Grauso, F.; Luisi, A.; Schettino, M.T.; Torella, M.; Colacurci, N. Adding agnus castus and magnolia to soy isoflavones relieves sleep disturbances besides postmenopausal vasomotor symptoms-long term safety and effectiveness. Nutrients 2017, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Yang, S.; Zhang, B.; Lu, Y. Certification of reference materials for analysis of isoflavones genistin and genistein in soy products. Anal. Methods 2016, 8, 89–96. [Google Scholar] [CrossRef]
- Atteritano, M.; Mazzaferro, S.; Bitto, A.; Cannata, M.L.; D’Anna, R.; Squadrito, F.; Macri, I.; Frisina, A.; Frisina, N.; Bagnato, G. Genistein effects on quality of life and depression symptoms in osteopenic postmenopausal women: A 2-year randomized, double-blind, controlled study. Osteoporos. Int. 2014, 25, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Furlong, O.; Parr, H.; Hodge, S.J.; Slevin, M.M.; Simpson, E.E.; McSorley, E.M.; McCormack, J.M.; Magee, P.J. Consumption of soy drink has no drink on cognitive function but may alleviate vasomotor symptoms in post-menopausal women—A randomised trial. Eur. J. Nutr. 2020, 59, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahdadi, K.J.; Tostes, R.C.; Webb, R.C. Female sexual dysfunction: Therapeutic options and experimental challenges. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Basson, R.; Gilks, T. Women’s sexual dysfunction associated with psychiatric disorders and their treatment. Womens Health 2018, 14, 1745506518762664. [Google Scholar] [CrossRef] [Green Version]
- Jaafarpour, M.; Khani, A.; Khajavikhan, J.; Suhrabi, Z. Female sexual dysfunction: Prevalence and risk factors. J. Clin. Diagn. Res. 2013, 7, 2877–2880. [Google Scholar] [PubMed]
- Zhang, J.; Zhu, Y.; Pan, L.; Xia, H.; Ma, J.; Zhang, A. Soy isoflavone improved female sexual dysfunction of mice via endothelial nitric oxide synthase pathway. Sex. Med. 2019, 7, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Daily, J.W.; Ko, B.S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol decreases hot flashes in postmenopausal women: A systematic review and meta-analysis of randomized clinical trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Hachul, H.; Brandao, L.C.; D’Almeida, V.; Bittencourt, L.R.; Baracat, E.C.; Tufik, S. Isoflavones decrease insomnia in postmenopause. Menopause 2011, 18, 178–184. [Google Scholar] [CrossRef]
- Burt Solorzano, C.M.; McCartney, C.R. Obesity and the pubertal transition in girls and boys. Reproduction 2010, 140, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Desmawati, D.; Sulastri, D. A phytoestrogens and their health effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Park, M.J. Effects of phytoestrogen on sexual development. Korean J. Pediatr. 2012, 55, 265–271. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macon, M.B.; Fenton, S.E. Endocrine disruptors and the breast: Early life effects and later life disease. J. Mammary Gland Biol. Neoplasia 2013, 18, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.X.; Zhang, B.; Li, L.L.; Xiao, C.W.; Fan, J.X.; Geng, M.M.; Yin, Y.L. Effects of soybean isoflavones on reproductive parameters in Chinese mini-pig boars. J. Anim. Sci. Biotechnol. 2012, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woclawek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Wasniewski, T.; Skarzynski, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 2013, 650984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinsdale, E.C.; Ward, W.E. Early exposure to soy isoflavones and effects on reproductive health: A review of human and animal studies. Nutrients 2010, 2, 1156–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinai, T.; Ben-Avraham, S.; Guelmann-Mizrahi, I.; Goldberg, M.R.; Naugolni, L.; Askapa, G.; Katz, Y.; Rachmiel, M. Consumption of soy-based infant formula is not associated with early onset of puberty. Eur. J. Nutr. 2019, 58, 681–687. [Google Scholar] [CrossRef]
- Duitama, S.M.; Zurita, J.; Cordoba, D.; Duran, P.; Ilag, L.; Mejia, W. Soy protein supplement intake for 12 months has no effect on sexual maturation and may improve nutritional status in pre-pubertal children. J. Paediatr. Child Health 2018, 54, 997–1004. [Google Scholar] [CrossRef]
- Ronis, M.J.; Gomez-Acevedo, H.; Shankar, K.; Sharma, N.; Blackburn, M.; Singhal, R.; Mercer, K.E.; Badger, T.M. EB 2017 Article: Soy protein isolate feeding does not result in reproductive toxicity in the pre-pubertal rat testis. Exp. Biol. Med. 2018, 243, 695–707. [Google Scholar] [CrossRef]
- Bernardo, B.D.; Brandt, J.Z.; Grassi, T.F.; Silveira, L.T.; Scarano, W.R.; Barbisan, L.F. Genistein reduces the noxious effects of in utero bisphenol A exposure on the rat prostate gland at weaning and in adulthood. Food Chem. Toxicol. 2015, 84, 64–73. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberg, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Tokinobu, A.; Arakawa, M. Maternal consumption of soy and isoflavones during pregnancy and risk of childhood behavioural problems: The Kyushu Okinawa Maternal and Child Health Study. Int. J. Food Sci. Nutr. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Wu, A.H.; Fears, T.; Nomura, A.M.; West, D.W.; Kolonel, L.N.; Pike, M.C.; Hoover, R.N.; Ziegler, R.G. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Upson, K.; Sathyanarayana, S.; Scholes, D.; Holt, V.L. Early-life factors and endometriosis risk. Fertil. Steril. 2015, 104, 964–971.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gencel, V.B.; Benjamin, M.M.; Bahou, S.N.; Khalil, R.A. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev. Med. Chem. 2012, 12, 149–174. [Google Scholar] [CrossRef]
- Cavallini, D.C.; Manzoni, M.S.; Bedani, R.; Roselino, M.N.; Celiberto, L.S.; Vendramini, R.C.; de Valdez, G.; Abdalla, D.S.; Pinto, R.A.; Rosetto, D.; et al. Probiotic soy product supplemented with isoflavones improves the lipid profile of moderately hypercholesterolemic men: A randomized controlled trial. Nutrients 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Kladna, A.; Berczynski, P.; Kruk, I.; Piechowska, T.; Aboul-Enein, H.Y. Studies on the antioxidant properties of some phytoestrogens. Luminescence 2016, 31, 1201–1206. [Google Scholar] [CrossRef]
- Rufer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food. Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Tronina, T.; Poplonski, J.; Bartmanska, A. Flavonoids as phytoestrogenic components of hops and beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Stochmal, A.; Pecetti, L. Isoflavone content in subterranean clover germplasm from Sardinia. Chem. Biodivers. 2016, 13, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Galazka-Czarnecka, I.; Brzozowska, E.; Grzelczyk, J.; Mostowski, R.; Zyzelewicz, D.; Ceron-Carrasco, J.P.; Perez-Sanchez, H. Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modelling. Food Chem. 2018, 245, 324–336. [Google Scholar] [CrossRef]
- Borras, C.; Gambini, J.; Gomez-Cabrera, M.C.; Sastre, J.; Pallardo, F.V.; Mann, G.E.; Vina, J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFkeppaB. FASEB J. 2006, 20, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, Y.; Yang, T.; Xue, Y.; Wang, P. Isoflavone intake inhibits the development of 7,12-dimethylbenz(a)anthracene(DMBA)-induced mammary tumors in normal and ovariectomized rats. J. Clin. Biochem. Nutr. 2014, 54, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006, 79, 1578–1584. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur. J. Pharmacol. 2011, 650, 694–702. [Google Scholar] [CrossRef]
- Kasapoglu, M.; Özben, T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp. Gerontol. 2001, 36, 209–220. [Google Scholar] [CrossRef]
- Gou, Z.; Jiang, S.; Zheng, C.; Tian, Z.; Lin, X. Equol inhibits LPS-induced oxidative stress and enhances the immune response in chicken HD11 macrophages. Cell. Physiol. Biochem. 2015, 36, 611–621. [Google Scholar] [CrossRef]
- Qian, K.; Gao, A.J.; Zhu, M.Y.; Shao, H.X.; Jin, W.J.; Ye, J.Q.; Qin, A.J. Genistein inhibits the replication of avian leucosis virus subgroup J in DF-1 cells. Virus Res. 2014, 192, 114–120. [Google Scholar] [CrossRef]
- Andres, A.; Donovan, S.M.; Kuhlenschmidt, M.S. Soy isoflavones and virus infections. J. Nutr. Biochem. 2009, 20, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Sithisarn, P.; Michaelis, M.; Schubert-Zsilavecz, M.; Cinatl Jr, J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013, 97, 41–48. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Ohya, Y.; Miyamoto, S.; Matsunaga, I.; Yoshida, T.; Hirota, Y.; Oda, H. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: The Osaka Maternal and Child Health Study. J. Allergy Clin. Immunol. 2005, 115, 1176–1183. [Google Scholar] [CrossRef]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Marini, H.; Polito, F.; Adamo, E.B.; Bitto, A.; Squadrito, F.; Benvenga, S. Update on genistein and thyroid: An overall message of safety. Front. Endocrinol. (Lausanne) 2012, 3, 94. [Google Scholar] [CrossRef] [Green Version]
- Benvenga, S.; Ducharme, M.; Yue, C.S.; Colucci, P. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur. Endocrinol. 2010, 9, 40–47. [Google Scholar]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [Green Version]
- Messina, M.; Mejia, S.B.; Cassidy, A.; Duncan, A.; Kurzer, M.; Nagato, C.; Ronis, M.; Rowland, I.; Sievenpiper, J.; Barnes, S. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: A technical review of the observational and clinical data. Crit. Rev. Food Sci. Nutr. 2021, 1–57. [Google Scholar] [CrossRef]
- Setchell, K.D.R. The history and basic science development of soy isoflavones. Menopause 2017, 24, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Rachon, D.; Menche, A.; Vortherms, T.; Seidlova-Wuttke, D.; Wuttke, W. Effects of dietary equol administration on the mammary gland in ovariectomized Sprague-Dawley rats. Menopause 2008, 15, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Adgent, M.A.; Wegienka, G.; Baird, D.D. Soy-based infant formula feeding and menstrual pain in a cohort of women aged 23-35 years. Hum. Reprod. 2019, 34, 148–154. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Miura, T.; Hanaoka, T.; Iwasaki, M.; Sasaki, H.; Tanaka, T.; Nakao, H.; Katoh, T.; Ikenoue, T.; Kabuto, M.; et al. Effect of soy isoflavones on endometriosis: Interaction with estrogen receptor 2 gene polymorphism. Epidemiology 2007, 18, 402–408. [Google Scholar] [CrossRef]
- Yamamoto, A.; Johnstone, E.B.; Bloom, M.S.; Huddleston, H.G.; Fujimoto, V.Y. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J. Assist. Reprod. Genet. 2017, 34, 765–774. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Chen, J.L.; Liu, Q.; Zhang, Y.; Zeng, H.; Zhao, Y. Soy intake is associated with lower endometrial cancer risk: A systematic review and meta-analysis of observational studies. Medicine 2015, 94, e2281. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Meyer, W.R.; Lessey, B.A.; Oi, R.H.; DeWire, R.E.; Fritz, M.A. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: A pilot trial. Menopause 2003, 10, 456–464. [Google Scholar] [CrossRef]
- Unfer, V.; Casini, M.L.; Costabile, L.; Mignosa, M.; Gerli, S.; Di Renzo, G.C. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: A randomized trial. J. Soc. Gynecol. Investig. 2004, 11, 323–328. [Google Scholar] [CrossRef]
- Unfer, V.; Casini, M.L.; Costabile, L.; Mignosa, M.; Gerli, S.; Di Renzo, G.C. Endometrial effects of long-term treatment with phytoestrogens: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2004, 82, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Lule, V.K.; Malik, R.K.; Tomar, S.K. Soy bioactive components in functional perspective: A review. Int. J. Food Prop. 2016, 19, 2550–2574. [Google Scholar] [CrossRef] [Green Version]
- Song, W.O.; Chun, O.K.; Hwang, I.; Shin, H.S.; Kim, B.G.; Kim, K.S.; Lee, S.Y.; Shin, D.; Lee, S.G. Soy isoflavones as safe functional ingredients. J. Med. Food 2007, 10, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.H. Estimated dietary isoflavone intake among Korean adults. Nutr. Res. Pract. 2007, 1, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. https://doi.org/10.3390/antiox10071064
Kim I-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants. 2021; 10(7):1064. https://doi.org/10.3390/antiox10071064
Chicago/Turabian StyleKim, Il-Sup. 2021. "Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans" Antioxidants 10, no. 7: 1064. https://doi.org/10.3390/antiox10071064