New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. E. histolytica Trophozoites
2.2. Neutrophil Isolation
2.3. NET Quantitation Assay
2.4. NET Visualization
2.5. Intracellular ROS Quantitation in Neutrophils
2.6. Mitochondrial ROS Quantitation in Neutrophils
2.7. ROS Quantitation in E. histolytica Trophozoites
2.8. ROS Scavenging from Amoebic Trophozoites
2.9. Visualization of Amoebas-Derived ROS
2.10. Detection of MPO Activity
2.11. Statistical Analysis
3. Results
3.1. E. histolytica Trophozoites Induce NETosis in a Dose-Dependent Manner
3.2. NETosis Induced by E. histolytica Trophozoites Is Independent of Neutrophil’s ROS
3.3. Dead Trophozoites Do Not Induce NET Release and Contain Scarce ROS
3.4. Amoebas Derived ROS Are Required for NETosis
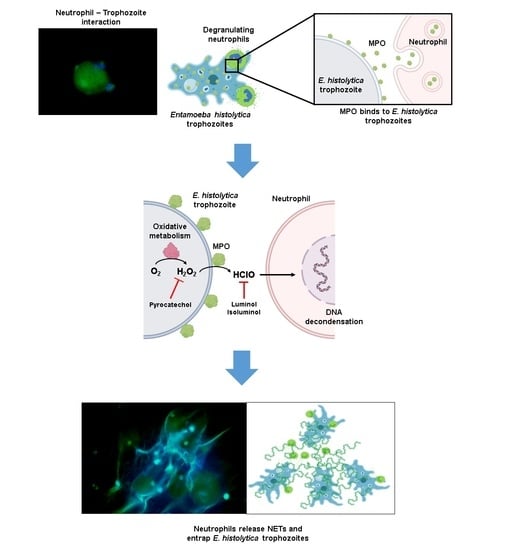
3.5. MPO Activity Is Detected Early during Neutrophil-Amoeba Interaction
3.6. MPO Activity Is Required for NETosis Induced by E. histolytica
3.7. MPO Is Detected on the Surface of Amoebic Trophozoites Early after Contact with Neutrophils and Its Activity Is Required for Trophozoite-Induced NETosis
3.8. E. histolytica-Induced NETosis Occurs Independently of Mitochondrial Derived ROS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkmann, V. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Ramos-Kichik, V.; Mondragón-Flores, R.; Mondragón-Castelán, M.; Gonzalez-Pozos, S.; Muñiz-Hernandez, S.; Rojas-Espinosa, O.; Chacón-Salinas, R.; Estrada-Parra, S.; Estrada-García, I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis 2009, 89, 29–37. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Heesemann, L.; Wagener, J.; Marcos, V.; Hartl, D.; Loeffler, J.; Heesemann, J.; Ebel, F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010, 12, 928–936. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Davis, R.P.; Kim, S.J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, C.; Zhao, M.H.; Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015, 181, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tydén, H.; Lood, C.; Truedsson, L.; Bengtsson, A.A.; Blom, A.M. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012, 188, 3522–3531. [Google Scholar] [CrossRef] [Green Version]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil extracellular traps: Mechanisms of formation and role in health and disease. Biochemistry 2014, 79, 1286–1296. [Google Scholar] [CrossRef]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New insights into neutrophil extracellular traps: Mechanisms of formation and role in inflammation. Front. Immunol. 2016, 7, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollberger, G.; Tilley, D.O.; Zychlinsky, A. Neutrophil extracellular traps: The biology of chromatin externalization. Dev. Cell 2018, 44, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Rocha, D.; Thomaz-Tobias, M.; Diniz, L.F.A.; Souza, P.S.S.; Pinge-Filho, P.; Toledo, K.A. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating toll-like receptors. PLoS ONE 2015, 10, e0139569. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Y.; Weng, C.L.; Jheng, M.J.; Kan, H.W.; Hsieh, S.T.; Liu, F.T.; Wu-Hsieh, B.A. Candida albicans triggers NADPH oxidaseindependent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019, 15, e1008096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, M.J.; Lalwani, P.; Krautkrämer, E.; Peters, T.; Scharffetter-Kochanek, K.; Krüger, R.; Hofmann, J.; Seeger, K.; Krüger, D.H.; Schönrich, G. Β2 Integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014, 211, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Alemán, O.R.; Mora, N.; Cortes-Vieyra, R.; Uribe-Querol, E.; Rosales, C. Differential use of human neutrophil Fc γ receptors for inducing neutrophil extracellular trap formation. J. Immunol. Res. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.; McCarty, O.J.T. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am. J. Physiol. Cell Physiol. 2013, 305, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSouza-Vieira, T.; Guimarães-Costa, A.; Rochael, N.C.; Lira, M.N.; Nascimento, M.T.; Lima-Gomez, P.D.S.; Mariante, R.M.; Persechini, P.M.; Saraiva, E.M. Neutrophil extracellular traps release induced by Leishmania: Role of PI3Kγ, ERK, PI3Kσ, PKC, and [Ca2+]. J. Leukoc. Biol. 2016, 100, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Thiama, H.R.; Wong, S.L.; Qiu, R.; Kittisopikul, M.; Vahabikashi, A.; Goldman, A.E.; Goldman, R.D.; Wagner, D.D.; Waterman, C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. USA 2020, 117, 7326–7337. [Google Scholar] [CrossRef] [Green Version]
- Neubert, E.; Meyer, D.; Rocca, F.; Günay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schön, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [Green Version]
- Nauseef, W.M.; Kubes, P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016, 18, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.-D. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef] [Green Version]
- de Bont, C.M.; Koopman, W.J.H.; Boelens, W.C.; Pruijn, G.J.M. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Gerretsen, J.; Boeltz, S.; Munoz, L.E.; Herrmann, M.; Pickkers, P.; Hilbrands, L.B.; Van Der Vlag, J. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann. Rheum. Dis. 2018, 77, 1790–1798. [Google Scholar] [CrossRef]
- Patel, S.; Kumar, S.; Jyoti, A.; Srinag, B.S.; Keshari, R.S.; Saluja, R.; Verma, A.; Mitra, K.; Barthwal, M.K.; Krishnamurthy, H.; et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide Biol. Chem. 2010, 22, 226–234. [Google Scholar] [CrossRef]
- Akong-Moore, K.; Chow, O.A.; von Köckritz-Blickwede, M.; Nizet, V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS ONE 2012, 7, e42984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBee, M.E.; Chionh, Y.H.; Sharaf, M.L.; Ho, P.; Cai, M.W.L.; Dedon, P.C. Production of superoxide in bacteria is stress and cell state-dependent: A gating-optimized flow cytometry method that minimizes ROS measurement artifacts with fluorescent dyes. Front. Microbiol. 2017, 8, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, E.F.; Muth, A.; Mondal, S.; Herzig, A.; Kru, R.; Thompson, P.R.; Brinkmann, V.; Von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; de la Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; León, C.M.; Fonseca, J.; Reyes, P.; Moncada, L.; Olivera, M.J.; Ramírez, J.D. Molecular epidemiology of Entamoeba: First description of Entamoeba moshkovskii in a rural area from central Colombia. PLoS ONE 2015, 10, e0140302. [Google Scholar] [CrossRef] [Green Version]
- Al-Areeqi, M.A.; Sady, H.; Al-Mekhlafi, H.M.; Anuar, T.S.; Al-Adhroey, A.H.; Atroosh, W.M.; Dawaki, S.; Elyana, F.N.; Nasr, N.A.; Ithoi, I.; et al. First molecular epidemiology of Entamoeba histolytica, E. dispar and E. moshkovskii infections in Yemen: Different species-specific associated risk factors. Trop. Med. Int. Health 2017, 22, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazi, M.A.; Patel, T.A.; El-Deek, B.S. Prevalence and characters of Entamoeba histolytica infection in Saudi infants and children admitted with diarrhea at 2 main hospitals at south Jeddah: A re-emerging serious infection with unusual presentation. Braz. J. Infect. Dis. 2013, 17, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Rodríguez, R.; Gutiérrez-Meza, M.; Jarillo-Luna, R.A.; Drago-Serrano, M.E.; Abarca-Rojano, E.; Ventura-Juárez, J.; Cárdenas-Jaramillo, L.M.; Pacheco-Yepez, J. A review of the proposed role of neutrophils in rodent amebic liver abscess models. Parasite 2016, 6, 14. [Google Scholar] [CrossRef]
- Velazquez, C.; Shibayama-Salas, M.; Aguirre-Garcia, J.; Tsutsumi, V.; Calderon, J. Role of neutrophils in innate resistance to Entamoeba histolytica liver infection in mice. Parasite Immunol. 1998, 20, 255–262. [Google Scholar] [CrossRef]
- Olivos-García, A.; Carrero, J.C.; Ramos, E.; Nequiz, M.; Tello, E.; Montfort, I.; Pérez-Tamayo, R. Late experimental amebic liver abscess in hamster is inhibited by cyclosporine and N-acetylcysteine. Exp. Mol. Pathol. 2007, 82, 310–315. [Google Scholar] [CrossRef]
- Díaz-Godínez, C.; Fonseca, Z.; Néquiz, M.; Laclette, J.P.; Rosales, C. Entamoeba histolytica trophozoites induce a rapid non-classical NETosis mechanism independent of NOX2-derived reactive oxygen species and PAD4 activity. Front. Cell. Infect. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- García–García, E.; Uribe-Querol, E.; Rosales, C. A simple and efficient method to detect nuclear factor activation in human neutrophils by flow cytometry. J. Vis. Exp. 2013, 74, 50410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Godínez, C.; Martínez-Flores, A.; Argüello-García, R.; Olivos-García, A.; Néquiz-Avendaño, M.; Carrero, J.C. Role of extracellular traps promoted by intestinal parasites. Relationship with virulence. In Eukaryome Impact on Human Intestine Homeostasis and Mucosal Immunology, 1st ed.; Guillén, N., Ed.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 171–192. [Google Scholar]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, E.E.; Salaiza, N.; Pulido, J.; Rodríguez, M.C.; Díaz-Godínez, C.; Laclette, J.P.; Becker, I.; Carrero, J.C. Entamoeba histolytica trophozoites and lipopeptidophosphoglycan trigger human neutrophil extracellular traps. PLoS ONE 2016, 11, e158979. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, Z.; Díaz-Godínez, C.; Mora, N.; Alemán, O.R.; Uribe-Querol, E.; Carrero, J.C.; Rosales, C. Entamoeba histolytica induce signaling via Raf/MEK/ERK for neutrophil extracellular trap (NET) formation. Front. Cell. Infect. Microbiol. 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Z.; Uribe-Querol, E.; Díaz-Godínez, C.; Carrero, J.C.; Rosales, C. Pathogenic Entamoeba histolytica, but not Entamoeba dispar, induce neutrophil extracellular trap (NET) formation. J. Leukoc. Biol. 2019, 105, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.S.A.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef] [Green Version]
- Rochael, N.C.; Guimarães-Costa, A.B.; Nascimento, M.T.C.; Desouza-Vieira, T.S.; Oliveira, M.P.; Garciae Souza, L.F.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci. Rep. 2015, 5, 18302. [Google Scholar] [CrossRef] [Green Version]
- Leishmaniasis. Available online: https://www.cdc.gov/parasites/leishmaniasis/ (accessed on 13 January 2021).
- Chagas Disease. Available online: https://www.cdc.gov/parasites/chagas/index.html (accessed on 13 January 2021).
- Toxoplasmosis. Available online: https://www.cdc.gov/dpdx/toxoplasmosis/index.html (accessed on 13 January 2021).
- Lima, T.S.; Gov, L.; Lodoen, M.B. Evasion of human neutrophil-mediated host defense during Toxoplasma gondii infection. mBio 2018, 9, e02027-17. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.D.; Hay, C.; Henard, C.A.; Popov, V.; Garg, N.J.; Soong, L. Leishmania amazonensis amastigotes trigger neutrophil activation but resist neutrophil microbicidal mechanisms. Infect. Immun. 2013, 81, 3966–3974. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, M.F.; de Almeida, V.D.; de Souza, L.M.S.; Paiva, D.C.C.; Andrade, C.D.M.; de Medeiros Fernandes, T.A.A. Involvement of neutrophils in Chagas disease pathology. Parasite Immunol. 2018, 40, e12593. [Google Scholar] [CrossRef] [Green Version]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Röhm, M.; Grimm, M.J.; D’Auria, A.C.; Almyroudis, N.G.; Segal, B.H.; Urban, C.F. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect. Immun. 2014, 82, 1766–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.J.; Kernien, J.F.; Hoyer, A.R.; Nett, J.E. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci. Rep. 2017, 7, 13065. [Google Scholar] [CrossRef]
- Khan, M.A.; Philip, L.M.; Cheung, G.; Vadakepeedika, S.; Grasemann, H.; Sweezey, N.; Palaniyar, N. Regulating NETosis: Increasing pH promotes NADPH oxidase-dependent NETosis. Front. Med. 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Arbo, A.; Hoefsloot, M.; Ignacio-Santos, I. Entamoeba histolytica inhibits the respiratory burst of polymorphonuclear leukocytes. Arch. Investig. Med. 1990, 21, 57–61. [Google Scholar]
- Zhou, Y.; An, L.L.; Chaerkady, R.; Mittereder, N.; Clarke, L.; Cohen, T.S.; Chen, B.; Hess, S.; Sims, G.P.; Mustelin, T. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 2018, 8, 15228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiducci, E.; Lemberg, C.; Küng, N.; Schraner, E.; Theocharides, A.P.A.; LeibundGut-Landmann, S. Candida albicans-induced NETosis is independent of peptidylarginine deiminase 4. Front. Immunol. 2018, 9, 1573. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; Olivos-García, A.; Saavedra, E.; Nequiz, M.; Sánchez, E.C.; Tello, E.; El-Hafidi, M.; Saralegui, A.; Pineda, E.; Delgado, J.; et al. Entamoeba histolytica: Oxygen resistance and virulence. Int. J. Parasitol. 2009, 39, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.S.; Dutta, S.; Raha, S. Hydrogen peroxide-induced apoptosis-like cell death in Entamoeba histolytica. Parasitol. Int. 2010, 59, 166–172. [Google Scholar] [CrossRef]
- Kast, J.; Klockenbusch, C. Optimization of formaldehyde cross-linking for protein interaction analysis of non-tagged integrin β 1. J. Biomed. Biotechnol. 2010, 2010, 927585. [Google Scholar] [CrossRef] [Green Version]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef]
- Olivos-García, A.; Saavedra, E.; Nequiz, M.; Santos, F.; Luis-García, E.R.; Gudiño, M.; Pérez-Tamayo, R. The oxygen reduction pathway and heat shock stress response are both required for Entamoeba histolytica pathogenicity. Curr. Genet. 2016, 62, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, T.; Mller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2012, 3, 424. [Google Scholar] [CrossRef] [Green Version]
- Van Der Veen, B.S.; De Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Björnsdottir, H.; Welin, A.; Michaëlsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- de Souza, C.N.; Breda, L.C.D.; Khan, M.A.; de Almeida, S.R.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline pH promotes NADPH oxidase-independent neutrophil extracellular trap formation: A matter of mitochondrial reactive oxygen species generation and citrullination and cleavage of histone. Front. Immunol. 2018, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Bernal, C.; Kirschning, C.J.; Rosenstein, Y.; Rocha, L.M.; Rios-Sarabia, N.; Espinosa-Cantellano, M.; Becker, I.; Estrada, I.; Salazar-Gonzalez, R.M.; Lopez-Macias, C.; et al. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol 2005, 27, 127–137. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [Green Version]
- Davidsson, L.; Rudin, A.D.; Klose, F.P.S.; Buck, A.; Björkman, L.; Christenson, K.; Bylund, J. In vivo transmigrated human neutrophils are highly primed for intracellular radical production induced by monosodium urate crystals. Int. J. Mol. Sci. 2020, 21, 3750. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Bystrzycka, W.; Cieloch, A.; Glodkowska-Mrowka, E.; Jankowska-Steifer, E.; Heropolitanska-Pliszka, E.; Skrobot, A.; Muchowicz, A.; Ciepiela, O.; Wachowska, M.; et al. Nitric oxide and peroxynitrite trigger and enhance release of neutrophil extracellular traps. Cell. Mol. Life Sci. 2020, 77, 3059–3075. [Google Scholar] [CrossRef] [Green Version]
- Pacheco–Yépez, J.; Rivera-Aguilar, V.; Barbosa-Cabrera, E.; Rojas Hernández, S.; Jarillo-Luna, R.A.; Campos-Rodríguez, R. Myeloperoxidase binds to and kills Entamoeba histolytica trophozoites. Parasite Immunol. 2011, 33, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jancinová, V.; Drábiková, K.; Nosál, R.; Racková, L.; Májeková, M.; Holománová, D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox Rep. 2006, 11, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.J.; Cooper, P.R.; Ling, M.R.; Wright, H.J.; Huissoon, A.; Chapple, I.L.C. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2012, 167, 261–268. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. Myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Godínez, C.; Jorge-Rosas, J.F.; Néquiz, M.; Martínez-Calvillo, S.; Laclette, J.P.; Rosales, C.; Carrero, J.C. New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity. Antioxidants 2021, 10, 974. https://doi.org/10.3390/antiox10060974
Díaz-Godínez C, Jorge-Rosas JF, Néquiz M, Martínez-Calvillo S, Laclette JP, Rosales C, Carrero JC. New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity. Antioxidants. 2021; 10(6):974. https://doi.org/10.3390/antiox10060974
Chicago/Turabian StyleDíaz-Godínez, César, Joshue Fabián Jorge-Rosas, Mario Néquiz, Santiago Martínez-Calvillo, Juan P. Laclette, Carlos Rosales, and Julio C. Carrero. 2021. "New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity" Antioxidants 10, no. 6: 974. https://doi.org/10.3390/antiox10060974