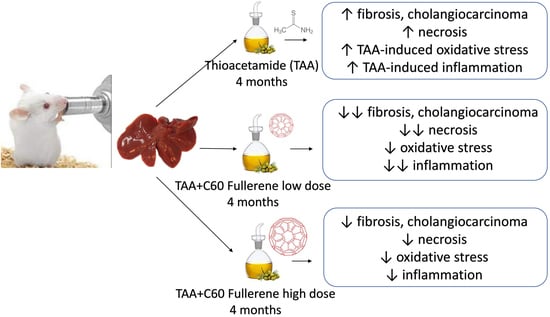
Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Preparation
2.3. Tissue Histology Analysis
2.4. Oxidative Status Analysis
2.5. Western Immunoblotting
2.5.1. Preparation of the Whole Liver Homogenates and Liver Nuclear Protein Fractions
2.5.2. Western Immunoblot Analysis
2.6. Biochemical Analysis
2.7. Comet Assay
2.8. Gut Microbiome Analysis
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stine, J.G.; Lewis, J.H. Current and future directions in the treatment and prevention of drug-induced liver injury: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 517–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Grebinyk, A.; Prylutska, S.; Buchelnikov, A.; Tverdokhleb, N.; Grebinyk, S.; Evstigneev, M.; Matyshevska, O.; Cherepanov, V.; Prylutskyy, Y.; Yashchuk, V.; et al. C60 Fullerene as an Effective Nanoplatform of Alkaloid Berberine Delivery into Leukemic Cells. Pharmaceutics 2019, 11, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franskevych, D.; Palyvoda, K.; Petukhov, D.; Prylutska, S.; Grynyuk, I.; Schuetze, C.; Drobot, L.; Matyshevska, O.; Ritter, U. Fullerene C60 Penetration into Leukemic Cells and Its Photoinduced Cytotoxic Effects. Nanoscale Res. Lett. 2017, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Takada, H.; Ito, S.; Matsubayashi, K.; Miwa, N.; Sawaguchi, T. Preclinical studies on safety of fullerene upon acute oral administration and evaluation for no mutagenesis. Toxicology 2006, 225, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Baati, T.; Bourasset, F.; Gharbi, N.; Njim, L.; Abderrabba, M.; Kerkeni, A.; Szwarc, H.; Moussa, F. The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials 2012, 33, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Mark, L.; Ohmacht, R.; Sharma, U. Olive oil as biocompatible solvent for pristine C60. Fuller. Nanotechnol. Carbon Nanostruct. 2007, 15, 311–314. [Google Scholar] [CrossRef]
- Cataldo, F. Interaction of C(60) fullerene with lipids. Chem. Phys. Lipids 2010, 163, 524–529. [Google Scholar] [CrossRef]
- Bhakuni, G.S.; Bedi, O.; Bariwal, J.; Deshmukh, R.; Kumar, P. Animal models of hepatotoxicity. Inflamm Res. 2016, 65, 13–24. [Google Scholar] [CrossRef]
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef] [Green Version]
- Zargar, S.; Wani, T.A.; Alamro, A.A.; Ganaie, M.A. Amelioration of thioacetamide-induced liver toxicity in Wistar rats by rutin. Int. J. Immunopathol. Pharm. 2017, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Elnfarawy, A.A.; Nashy, A.E.; Abozaid, A.M.; Komber, I.F.; Elweshahy, R.H.; Abdelrahman, R.S. Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats. Hum. Exp. Toxicol. 2021, 40, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 15th ed.; Oxford University Press: Oxford, UK, 2015; p. xxxviii. 905p. [Google Scholar]
- Maines, M.D.; Gibbs, P.E. 30 some years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem. Biophys. Res. Commun. 2005, 338, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.S.; Chen, H.P.; Yu, H.H.; Yan, Y.F.; Liao, Z.P.; Huang, Q.R. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell. Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Chu, H.; Duan, Y.; Schnabl, B. Gut microbiota in liver disease: Too much is harmful, nothing at all is not helpful either. Am. J. Physiol Gastrointest. Liver. Physiol. 2019, 316, G563–G573. [Google Scholar] [CrossRef]
- Brenner, D.A.; Paik, Y.H.; Schnabl, B. Role of Gut Microbiota in Liver Disease. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S25–S27. [Google Scholar] [CrossRef] [Green Version]
- Konopelko, L.A.; Krilov, A.N.; Lopushanskaya, E.M.; Popov, O.G. Adducts formation at fullerenes C60 and C70 dissolution in essential oils. Russ. J. Gen. Chem. 2014, 84, 205–208. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Halliwell, B.; Foyer, C.H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 1978, 139, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Gerard-Monnier, D.; Erdelmeier, I.; Regnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudiere, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Env. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pascoe, P.L.; Parry, J.M.; Dixon, D.R. Evaluation of the comet assay as a method for the detection of DNA damage in the cells of a marine invertebrate, Mytilus edulis L. (Mollusca: Pelecypoda). Mutat. Res. 1998, 399, 87–95. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; ISBN 3-900051-07-0. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. J. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Đurašević, S.; Nikolić, G.; Todorović, A.; Drakulić, D.; Pejić, S.; Martinović, V.; Mitić-Ćulafić, D.; Milić, D.; Kop, T.J.; Jasnić, N. Effects of fullerene C60 supplementation on gut microbiota and glucose and lipid homeostasis in rats. Food Chem. Toxicol. 2020, 140, 111302. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Piotrovsky, L.B.; Eropkin, M.Y.; Eropkina, E.M.; Dumpis, M.A.; Kiselev, O.I. Biological Effects in Cell Cultures of Fullerene C60: Dependence on Aggregation State. In Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Cataldo, F., Da Ros, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 1. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đurašević, S.; Nikolić, G.; Zaletel, I.; Grigorov, I.; Memon, L.; Mitić-Ćulafić, D.; Vujović, P.; Đorđević, J.; Todorović, Z. Distinct effects of virgin coconut oil supplementation on the glucose and lipid homeostasis in non-diabetic and alloxan-induced diabetic rats. J. Funct. Foods 2020, 64, 103601. [Google Scholar] [CrossRef]
- Memon, A.; Pyao, Y.; Jung, Y.; Lee, J.I.; Lee, W.K. A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 5461. [Google Scholar] [CrossRef] [PubMed]
- De Minicis, S.; Kisseleva, T.; Francis, H.; Baroni, G.S.; Benedetti, A.; Brenner, D.; Alvaro, D.; Alpini, G.; Marzioni, M. Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Dig. Liver Dis. 2013, 45, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncalli, M.; Terracciano, L.; Di Tommaso, L.; David, E.; Colombo, M.; Gruppo Italiano Patologi Apparato, D. Societa Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology, I.d. Liver precancerous lesions and hepatocellular carcinoma: The histology report. Dig. Liver Dis. 2011, 43 (Suppl. 4), S361–S372. [Google Scholar] [CrossRef]
- Yeh, C.N.; Maitra, A.; Lee, K.F.; Jan, Y.Y.; Chen, M.F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis 2004, 25, 631–636. [Google Scholar] [CrossRef]
- Rajcic, D.; Brandt, A.; Jin, C.J.; Sanchez, V.; Engstler, A.J.; Jung, F.; Nier, A.; Baumann, A.; Bergheim, I. Exchanging dietary fat source with extra virgin olive oil does not prevent progression of diet-induced non-alcoholic fatty liver disease and insulin resistance. PLoS ONE 2020, 15, e0237946. [Google Scholar] [CrossRef] [PubMed]
- Kouka, P.; Tekos, F.; Papoutsaki, Z.; Stathopoulos, P.; Halabalaki, M.; Tsantarliotou, M.; Zervos, I.; Nepka, C.; Liesivuori, J.; Rakitskii, V.N.; et al. Olive oil with high polyphenolic content induces both beneficial and harmful alterations on rat redox status depending on the tissue. Toxicol. Rep. 2020, 7, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Ge, S.; Wu, X.; Xiong, Y.; Xie, J.; Liu, F.; Zhang, W.; Yang, L.; Zhang, S.; Lai, L.; Huang, J.; et al. HMGB1 Inhibits HNF1A to Modulate Liver Fibrogenesis via p65/miR-146b Signaling. DNA Cell Biol. 2020, 39, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [Green Version]
- Alva, N.; Panisello-Rosello, A.; Flores, M.; Rosello-Catafau, J.; Carbonell, T. Ubiquitin-proteasome system and oxidative stress in liver transplantation. World J. Gastroenterol. 2018, 24, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.P.; Colaco, A.A.; Oliveira, P.A. Animal models as a tool in hepatocellular carcinoma research: A Review. Tumour Biol. 2017, 39, 1010428317695923. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, S.; Ur Rehman, A.; Tiebosch, M.H.; Hannivoort, R.A.; Haijer, F.W.; Woudenberg, J.; van den Heuvel, F.A.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim. Biophys. Acta 2013, 1832, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Mari, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial Glutathione: Recent Insights and Role in Disease. Antioxidants 2020, 9, 909. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Ebaid, H.; Abdelmottaleb Moussa, S.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Al Tamimi, J. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective Effect of Aqueous Extract from the Leaves of Justicia tranquebariesis against Thioacetamide-Induced Oxidative Stress and Hepatic Fibrosis in Rats. Antioxidants 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de David, C.; Rodrigues, G.; Bona, S.; Meurer, L.; Gonzalez-Gallego, J.; Tunon, M.J.; Marroni, N.P. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol. Pathol. 2011, 39, 949–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Abusarah, J.; Bentz, M.; Benabdoune, H.; Rondon, P.E.; Shi, Q.; Fernandes, J.C.; Fahmi, H.; Benderdour, M. An overview of the role of lipid peroxidation-derived 4-hydroxynonenal in osteoarthritis. Inflamm. Res. 2017, 66, 637–651. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Rojo, A.I.; Salinas, M.; Martin, D.; Perona, R.; Cuadrado, A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J. Neurosci. 2004, 24, 7324–7334. [Google Scholar] [CrossRef] [Green Version]
- Moinova, H.R.; Mulcahy, R.T. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem. Biophys. Res. Commun. 1999, 261, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Gijbels, E.; Jonckheer, J.; De Waele, E.; Vinken, M. Cholestatic liver injury induced by food additives, dietary supplements and parenteral nutrition. Environ. Int. 2020, 136, 105422. [Google Scholar] [CrossRef] [PubMed]
- Schyman, P.; Printz, R.L.; Estes, S.K.; Boyd, K.L.; Shiota, M.; Wallqvist, A. Identification of the Toxicity Pathways Associated With Thioacetamide-Induced Injuries in Rat Liver and Kidney. Front. Pharm. 2018, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.Y. Physiological and histopathological study on the influence of Ocimum basilicum leaves extract on thioacetamide-induced nephrotoxicity in male rats. Saudi J. Biol. Sci. 2020, 27, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef] [PubMed]
- Rader, B.A. Alkaline Phosphatase, an Unconventional Immune Protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalles, J.P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Evivie, S.E.; Huo, G.C.; Igene, J.O.; Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr. Res. 2017, 61, 1318034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Bai, Y.; Zhang, G.; Liu, L.; Lai, C. Relationship between Dietary Fiber Fermentation and Volatile Fatty Acids' Concentration in Growing Pigs. Animals 2020, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. J. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iljazovic, A.; Roy, U.; Galvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]






| Groups | Application of TAA | Application of VOO without C60 | Application of VOO with Low C60 Dose | Application of VOO with High C60 Dose |
|---|---|---|---|---|
| Control | none | none | none | none |
| TAA | + | none | none | none |
| TAA+O | + | + | none | none |
| TAA+F1 | + | none | + | none |
| TAA+F2 | + | none | none | + |
| Groups | Control | TAA | TAA+O | TAA+F1 | TAA+F2 |
|---|---|---|---|---|---|
| Body mass AUC | 100.00 ± 1.16 | 80.36 ± 0.88 a | 78.80 ± 1.33 a | 85.17 ± 1.20 a | 74.56 ± 1.04 a,b,c,d |
| Food intake AUC | 100.00 ± 1.52 | 88.80 ± 1.77 a | 89.19 ± 2.17 a | 86.52 ± 1.53 a | 85.29 ± 1.66 a |
| Water intake AUC | 100.00 ± 1.74 | 78.70 ± 1.28 a | 77.77 ± 1.56 a | 75.03 ± 1.40 a | 66.81 ± 1.30 a,b,c,d |
| Glycaemia AUC | 100.00 ± 0.76 | 101.29 ± 0.83 | 101.37 ± 1.11 | 101.20 ± 1.20 | 107.30 ± 1.26 |
| VOO intake | – | – | 5.923 ± 0.127 | 5.805 ± 0.094 | 5.355 ± 0.115 |
| C60 intake | – | – | – | 1.146 ± 0.026 | 5.231 ± 0.126 d |
| C60 liver bioaccumulation | – | – | – | 21.04 ± 0.60 | 33.32 ± 0.98 d |
| Groups | Control | TAA | TAA+O | TAA+F1 | TAA+F2 |
|---|---|---|---|---|---|
| Parenchymal injury | 0.15 | 1.550 | 2.025 | 1.525 | 1.675 |
| Fatty Change | 0.25 | 1.250 | 2.375 | 1.500 | 1.875 |
| Fibrosis | 0 | 3.625 | 3.750 | 2.500 | 3.000 |
| Biliary injury | 0 | 0.662 | 0.850 | 0.525 | 0.775 |
| Cholangiocarcinoma | 0 | 75% | 100% | 50% | 75% |
| Inflammation | 0 | 0.844 | 0.900 | 0.731 | 0.794 |
| Groups | Control | TAA | TAA+O | TAA+F1 | TAA+F2 |
|---|---|---|---|---|---|
| SOD | 6.355 ± 0.320 | 8.623 ± 0.506 a | 11.623 ± 0.388 a,b | 6.049 ± 0.203 b,c | 5.970 ± 0.449 b,c |
| CAT | 71.815 ± 1.995 | 54.900 ± 2.614 a | 56.399 ± 4.623 a | 92.255 ± 2.352 a,b,c | 62.124 ± 3.931 d |
| GPx | 37.167 ± 1.846 | 48.911 ± 2.769 a | 48.265 ± 1.424 a | 35.263 ± 1.851 b,c | 29.688 ± 2.004 b,c |
| GR | 24.963 ± 1.686 | 39.859 ± 0.867 a | 44.164 ± 2.041 a | 45.784 ± 2.075 a | 46.939 ± 2.613 a |
| GST | 162.477 ± 11.425 | 325.490 ± 20.809 a | 549.839 ± 30.678 a,b | 550.760 ± 35.467 a,b | 516.204 ± 32.408 a,b |
| GSH | 37.938 ± 1.460 | 29.553 ± 1.227 a | 31.094 ± 1.351 a | 31.155 ± 1.222 a | 30.205 ± 2.228 a |
| HNE | 1.865 ± 0.126 | 1.757 ± 0.167 | 1.567 ± 0.133 | 1.421 ± 0.143 a | 1.391 ± 0.176 a |
| Groups | Control | TAA | TAA+O | TAA+F1 | TAA+F2 |
|---|---|---|---|---|---|
| AST | 177.38 ± 3.59 | 207.38 ± 5.91 a | 200.88 ± 4.43 a | 212.89 ± 4.22 a | 216.25 ± 6.19 a |
| ALT | 36.20 ± 2.19 | 53.25 ± 2.46 a | 52.75 ± 3.14 a | 59.88 ± 2.39 a | 61.88 ± 3.17 a |
| AP | 30.38 ± 2.04 | 164.50 ± 13.08 a | 302.50 ± 7.41 a,b | 262.38 ± 8.20 a,b,c | 300.75 ± 6.49 a,b,d |
| Comet | 2.754 ± 0.282 | 9.274 ± 0.717 a | 5.562 ± 0.392 a,b | 2.072 ± 0.137 b,c | 2.972 ± 0.220 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurašević, S.; Pejić, S.; Grigorov, I.; Nikolić, G.; Mitić-Ćulafić, D.; Dragićević, M.; Đorđević, J.; Todorović Vukotić, N.; Đorđević, N.; Todorović, A.; et al. Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes. Antioxidants 2021, 10, 911. https://doi.org/10.3390/antiox10060911
Đurašević S, Pejić S, Grigorov I, Nikolić G, Mitić-Ćulafić D, Dragićević M, Đorđević J, Todorović Vukotić N, Đorđević N, Todorović A, et al. Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes. Antioxidants. 2021; 10(6):911. https://doi.org/10.3390/antiox10060911
Chicago/Turabian StyleĐurašević, Siniša, Snežana Pejić, Ilijana Grigorov, Gorana Nikolić, Dragana Mitić-Ćulafić, Milan Dragićević, Jelena Đorđević, Nevena Todorović Vukotić, Neda Đorđević, Ana Todorović, and et al. 2021. "Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes" Antioxidants 10, no. 6: 911. https://doi.org/10.3390/antiox10060911