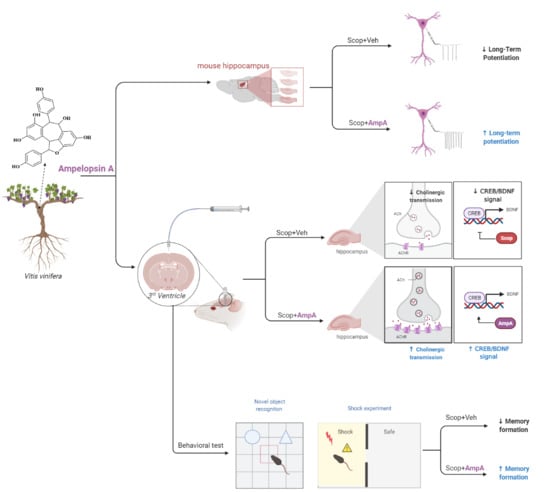
Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures and Plant Material
2.2. Spectroscopy of Isolated Ampelopsin A from the Stem Bark of V. vinifera
2.3. Slice Preparation and Electrophysiology
2.4. Animals
2.5. Surgical Procedure and Treatments
2.6. Behavioral Tests
2.6.1. Open Field Test
2.6.2. Novel Object Recognition Test
2.6.3. Passive Avoidance Test
2.7. ChAT Activity
2.8. Ach Level and AChE Activity
2.9. Quantitative Real-Time PCR Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Isolation and Determination of Compound from the Stem Bark of V. vinifera
3.2. Bath Application of Ampelopsin A Increases the Neuronal Excitability of Hippocampal Neurons
3.3. Administration of Ampelopsin A into the 3 V Increased Cognitive Memory Behaviors
3.3.1. Open Field Test
3.3.2. Novel Object Recognition Test
3.3.3. Passive Avoidance Test
3.4. Administration of Ampelopsin A Ameliorates Cholinergic Dysfunction
3.5. Administration of Amplopsin A Elevates BDNF-Related Signaling in the Hippocampus
3.6. Antioxidant and Anti-Apoptotic Effects on the Hippocampus by Ampelopsin A
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Drever, B.D.; Riedel, G.; Platt, B. The Cholinergic System and Hippocampal Plasticity. Behav. Brain Res. 2011, 221, 505–514. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic System during the Progression of Alzheimer’s Disease: Therapeutic Implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2020, 108352. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Sevilla, D.; Nunez, A.; Buno, W. Muscarinic Receptors, from Synaptic Plasticity to its Role in Network Activity. Neuroscience 2021, 456, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Shinoe, T.; Matsui, M.; Taketo, M.M.; Manabe, T. Modulation of Synaptic Plasticity by Physiological Activation of M1 Muscarinic Acetylcholine Receptors in the Mouse Hippocampus. J. Neurosci. 2005, 25, 11194–11200. [Google Scholar] [CrossRef] [Green Version]
- Klinkenberg, I.; Blokland, A. The Validity of Scopolamine as a Pharmacological Model for Cognitive Impairment: A Review of Animal Behavioral Studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal Memory Enhancing Activity of Pine Needle Extract against Scopolamine-induced Amnesia in a Mouse Model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef] [Green Version]
- Bekinschtein, P.; Cammarota, M.; Igaz, L.M.; Bevilaqua, L.R.; Izquierdo, I.; Medina, J.H. Persistence of Long-term Memory Storage Requires a Late Protein Synthesis- and BDNF- Dependent Phase in the Hippocampus. Neuron 2007, 53, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Martinez, S. A New Perspective on the Role of the CREB Family of Transcription Factors in Memory Consolidation via Adult Hippocampal Neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The Role of CREB and BDNF in Neurobiology and Treatment of Alzheimer’s Disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C.; Pawlus, A.D.; Merillon, J.M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Bordun, K.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, T.; Pawlus, A.D.; Iglesias, M.L.; Pedrot, E.; Waffo-Teguo, P.; Merillon, J.M.; Monti, J.P. Neuroprotective Properties of Resveratrol and Derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef]
- Lim, K.G.; Gray, A.I.; Anthony, N.G.; Mackay, S.P.; Pyne, S.; Pyne, N.J. Resveratrol and Its Oligomers: Modulation of Sphingolipid Metabolism and Signaling in Disease. Arch. Toxicol. 2014, 88, 2213–2232. [Google Scholar] [CrossRef] [Green Version]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Merillon, J.M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Temsamani, H.; Krisa, S.; Decossas-Mendoza, M.; Lambert, O.; Merillon, J.M.; Richard, T. Piceatannol and Other Wine Stilbenes: A Pool of Inhibitors against alpha-Synuclein Aggregation and Cytotoxicity. Nutrients 2016, 8, 367. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Yoo, M.Y.; Choi, C.W.; Cha, M.R.; Yon, G.H.; Kwon, D.Y.; Kim, Y.S.; Park, W.K.; Ryu, S.Y. A New Specific BACE-1 Inhibitor from the Stembark Extract of V. vinifera. Planta Med. 2009, 75, 537–540. [Google Scholar] [CrossRef]
- Pinho, B.R.; Ferreres, F.; Valentao, P.; Andrade, P.B. Nature as a Source of Metabolites with Cholinesterase-inhibitory Activity: An Approach to Alzheimer’s Disease Treatment. J. Pharm. Pharmacol. 2013, 65, 1681–1700. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Tosun, F.; Sener, B. Coumarin, Anthroquinone and Stilbene Derivatives with Anticholinesterase Activity. Z. Naturforsch. C J. Biosci. 2008, 63, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Namdaung, U.; Athipornchai, A.; Khammee, T.; Kuno, M.; Suksamrarn, S. 2-Arylbenzofurans from Artocarpus Lakoocha and Methyl Ether Analogs with Potent Cholinesterase Inhibitory Activity. Eur. J. Med. Chem. 2018, 143, 1301–1311. [Google Scholar] [CrossRef]
- Zga, N.; Papastamoulis, Y.; Toribio, A.; Richard, T.; Delaunay, J.C.; Jeandet, P.; Renault, J.H.; Monti, J.P.; Merillon, J.M.; Waffo-Teguo, P. Preparative Purification of Antiamyloidogenic Stilbenoids from Vitis vinifera (Chardonnay) Stems by Centrifugal Partition Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1000–1004. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D. Hypothalamic Programming of Systemic Ageing Involving IKK-beta, NF-kappaB and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Anchan, D.; Clark, S.; Pollard, K.; Vasudevan, N. GPR30 Activation Decreases Anxiety in the Open Field Test but not in the Elevated Plus Maze Test in Female Mice. Brain Behav. 2014, 4, 51–59. [Google Scholar] [CrossRef]
- Zhang, L.; Seo, J.H.; Li, H.; Nam, G.; Yang, H.O. The Phosphodiesterase 5 Inhibitor, KJH-1002, Reverses a Mouse Model of Amnesia by Activating a cGMP/cAMP Response Element Binding Protein Pathway and Decreasing Oxidative Damage. Br. J. Pharmacol. 2018, 175, 3347–3360. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Song, L.; Huang, C.; Zhang, W. P7C3 Attenuates the Scopolamine-Induced Memory Impairments in C57BL/6J Mice. Neurochem. Res. 2016, 41, 1010–1019. [Google Scholar] [CrossRef]
- Oshima, Y.; Ueno, Y.; Hikino, H.; Yang, L.L.; Yen, K.Y. Ampelopsin-a, Ampelopsin-B and Ampelopsin-C, New Oligostilbenes of Ampelopsis-Brevipedunculata Var Hancei. Tetrahedron 1990, 46, 5121–5126. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. Long-term Potentiation–A Decade of Progress? Science 1999, 285, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smythe, J.W.; Murphy, D.; Bhatnagar, S.; Timothy, C.; Costall, B. Muscarinic Antagonists are Anxiogenic in Rats Tested in the Black-white Box. Pharmacol. Biochem. Behav. 1996, 54, 57–63. [Google Scholar] [CrossRef]
- Rosenzweig-Lipson, S.; Thomas, S.; Barrett, J.E. Attenuation of the Locomotor Activating Effects of D-amphetamine, Cocaine, and Scopolamine by Potassium Channel Modulators. Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 853–872. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, J.; Ahmad, H.; Zhang, H.; Xu, Z.; Wang, T. Evaluation of Antioxidant Activities of Ampelopsin and Its Protective Effect in Lipopolysaccharide-induced Oxidative Stress Piglets. PLoS ONE 2014, 9, e108314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Shu, F.; Liang, X.; Chang, H.; Shi, L.; Peng, X.; Zhu, J.; Mi, M. Ampelopsin Induces Cell Growth Inhibition and Apoptosis in Breast Cancer Cells through ROS Generation and Endoplasmic Reticulum Stress Pathway. PLoS ONE 2014, 9, e89021. [Google Scholar] [CrossRef] [PubMed]
- Iliya, I.; Ali, Z.; Tanaka, T.; Iinuma, M.; Furusawa, M.; Nakaya, K.; Murata, J.; Darnaedi, D.; Matsuura, N.; Ubukata, M. Stilbene Derivatives from Gnetum gnemon Linn. Phytochemistry 2003, 62, 601–606. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. A New Class of Phytoalexins from Grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef]
- Bokel, M.; Diyasena, M.N.C.; Gunatilaka, A.A.L.; Kraus, W.; Sotheeswaran, S. Canaliculatol, an Antifungal Resveratrol Trimer from Stemonoporous-Canaliculatus. Phytochemistry 1988, 27, 377–380. [Google Scholar] [CrossRef]
- Kitanaka, S.; Ikezawa, T.; Yasukawa, K.; Yamanouchi, S.; Takido, M.; Sung, H.K.; Kim, I.H. (+)-Alpha-viniferin, an Anti-inflammatory Compound from Caragana chamlagu Root. Chem. Pharm. Bull. 1990, 38, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.X.; Terashima, K.; Takaya, Y.; Niwa, M. A Novel Oligostilbene Named (+)-viniferol A from the Stem of Vitis vinifera ‘Kyohou’. Tetrahedron 2001, 57, 2711–2715. [Google Scholar] [CrossRef]
- Valle, A.; Hoggard, N.; Adams, A.C.; Roca, P.; Speakman, J.R. Chronic Central Administration of Apelin-13 over 10 Days Increases Food Intake, Body Weight, Locomotor Activity and Body Temperature in C57BL/6 Mice. J. Neuroendocrinol. 2008, 20, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Treleaven, C.M.; Tamsett, T.; Fidler, J.A.; Taksir, T.V.; Cheng, S.H.; Shihabuddin, L.S.; Dodge, J.C. Comparative Analysis of Acid Sphingomyelinase Distribution in the CNS of Rats and Mice Following Intracerebroventricular Delivery. PLoS ONE 2011, 6, e16313. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Q.; Peng, S.Y.; Li, S.H.; Liu, S.Q.; Lv, Y.F.; Yang, N.; Yu, L.Y.; Deng, Y.H.; Zhang, Z.J.; Fang, M.S.; et al. Chronic Olanzapine Administration Causes Metabolic Syndrome through Inflammatory Cytokines in Rodent Models of Insulin Resistance. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Kim, M.S.; Jia, B.S.; Yan, J.Q.; Zuniga-Hertz, J.P.; Han, C.; Cai, D.S. Hypothalamic Stem Cells Control Ageing Speed Partly through Exosomal miRNAs. Nature 2017, 548. [Google Scholar] [CrossRef]
- Oh, S.Y.; Jang, M.J.; Choi, Y.H.; Hwang, H.; Rhim, H.; Lee, B.; Choi, C.W.; Kim, M.S. Central Administration of Afzelin Extracted from Ribes fasciculatum Improves Cognitive and Memory Function in a Mouse Model of Dementia. Sci. Rep. 2021, 11, 9182. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease: Getting on and Staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Giacobini, E. Cholinergic Function and Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 2003, 18, S1–S5. [Google Scholar] [CrossRef]
- Schegg, K.M.; Harrington, L.S.; Neilsen, S.; Zweig, R.M.; Peacock, J.H. Soluble and Membrane-bound Forms of Brain Acetylcholinesterase in Alzheimer’s Disease. Neurobiol. Aging 1992, 13, 697–704. [Google Scholar] [CrossRef]
- Santarpia, L.; Grandone, I.; Contaldo, F.; Pasanisi, F. Butyrylcholinesterase as a Prognostic Marker: A Review of the Literature. J. Cachexia Sarcopeni 2013, 4, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, Y.; Xing, S.; Liao, Q.; Xiong, B.; Wang, Y.; Lu, W.; He, S.; Feng, F.; Liu, W.; et al. Highly Potent and Selective Butyrylcholinesterase Inhibitors for Cognitive Improvement and Neuroprotection. J. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Kosak, U.; Brus, B.; Knez, D.; Sink, R.; Zakelj, S.; Trontelj, J.; Pislar, A.; Slenc, J.; Gobec, M.; Zivin, M.; et al. Development of an in vivo Active Reversible Butyrylcholinesterase Inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Xing, S.; Chen, Y.; Liao, Q.; Xiong, B.; He, S.; Lu, W.; Liu, Y.; Yang, H.; Li, Q.; et al. Discovery and Biological Evaluation of a Novel Highly Potent Selective Butyrylcholinsterase Inhibitor. J. Med. Chem. 2020, 63, 10030–10044. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.B., Jr.; Lindner, M.D.; Hogan, J.B.; Jones, K.M.; Markus, E.J. Scopolamine Induced Deficits in a Battery of Rat Cognitive Tests: Comparisons of Sensitivity and Specificity. Behav. Pharmacol. 2009, 20, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Nakae, K.; Nishimura, Y.; Ohba, S.; Akamatsu, Y. Migrastatin Acts as a Muscarinic Acetylcholine Receptor Antagonist. J. Antibiot. 2006, 59, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Spignoli, G.; Pepeu, G. Interactions between Oxiracetam, Aniracetam and Scopolamine on Behavior and Brain Acetylcholine. Pharmacol. Biochem. Behav. 1987, 27, 491–495. [Google Scholar] [CrossRef]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng Berry Aqueous Extract Prevents Scopolamine-induced Memory Impairment in Mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef] [Green Version]
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Pharmacological Evidence of Cholinergic Involvement in Adult Hippocampal Neurogenesis in Rats. Neuroscience 2006, 142, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Deng, Q.; Wu, H.; Bao, W.; Wang, D.; Yao, P.; Huang, F.; et al. Memory Impairment in Cognitively Impaired Aged Rats Associated with Decreased Hippocampal CREB Phosphorylation: Reversal by Procyanidins Extracted from the Lotus Seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.J.; Luo, D.; Li, L.; Tan, R.R.; Xu, Q.Q.; Qin, J.; Zhu, L.; Luo, N.C.; Xu, T.T.; Zhang, R.; et al. Ethyl Acetate Extract Components of Bushen-Yizhi Formula Provides Neuroprotection against Scopolamine-induced Cognitive Impairment. Sci. Rep. 2017, 7, 9824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich Fraction from Ishige foliacea Brown Seaweed Prevents the Scopolamine-induced Memory Impairment via Regulation of ERK-CREB-BDNF Pathway. J. Funct. Foods. 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Regue-Guyon, M.; Lanfumey, L.; Mongeau, R. Neuroepigenetics of Neurotrophin Signaling: Neurobiology of Anxiety and Affective Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 158, 159–193. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Vianna, M.R.M.; Depino, A.M.; Souza, T.M.E.; Pereira, P.; Szapiro, G.; Viola, H.; Pitossi, F.; Izquierdo, I.; Medina, J.H. BDNF-triggered Events in the Rat Hippocampus are Required for Both Short- and Long-term Memory Formation. Hippocampus 2002, 12, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Wang, H.L.; Wu, H.C.; Wei, C.L.; Lee, E.H. Brain-derived Neurotrophic Factor Antisense Oligonucleotide Impairs Memory Retention and Inhibits Long-term Potentiation in Rats. Neuroscience 1998, 82, 957–967. [Google Scholar] [CrossRef]
- Ou, L.C.; Gean, P.W. Transcriptional Regulation of Brain-derived Neurotrophic Factor in the Amygdala during Consolidation of Fear Memory. Mol. Pharmacol. 2007, 72, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Rattiner, L.M.; Davis, M.; French, C.T.; Ressler, K.J. Brain-derived Neurotrophic Factor and Tyrosine Kinase Receptor B Involvement in Amygdala-dependent Fear Conditioning. J. Neurosci. 2004, 24, 4796–4806. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vidal, L.E.; Do-Monte, F.H.; Sotres-Bayon, F.; Quirk, G.J. Hippocampal-prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology 2014, 39, 2161–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.; Dieppa-Perea, L.M.; Melendez, L.M.; Quirk, G.J. Induction of Fear Extinction with Hippocampal-infralimbic BDNF. Science 2010, 328, 1288–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.S. The Cellular and Molecular Processes Associated with Scopolamine-induced Memory Deficit: A Model of Alzheimer’s Biomarkers. Life Sci. 2019, 233. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.S.; Dreyfus, C.F.; Black, I.B.; Plummer, M.R. Brain-derived Neurotrophic Factor Rapidly Enhances Synaptic Transmission in Hippocampal Neurons via Postsynaptic Tyrosine Kinase Receptors. Proc. Natl. Acad. Sci. USA 1995, 92, 8074–8077. [Google Scholar] [CrossRef] [Green Version]
- Zakharenko, S.S.; Patterson, S.L.; Dragatsis, I.; Zeitlin, S.O.; Siegelbaum, S.A.; Kandel, E.R.; Morozov, A. Presynaptic BDNF Required for a Presynaptic but not Postsynaptic Component of LTP at Hippocampal CA1-CA3 Synapses. Neuron 2003, 39, 975–990. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Schuman, E.M. A Requirement for Local Protein Synthesis in Neurotrophin-induced Hippocampal Synaptic Plasticity. Science 1996, 273, 1402–1406. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Choi, Y.-H.; Han, Y.-E.; Oh, S.-J.; Lee, A.; Lee, B.; Magnan, R.; Ryu, S.Y.; Choi, C.W.; Kim, M.S. Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model. Antioxidants 2021, 10, 835. https://doi.org/10.3390/antiox10060835
Hong Y, Choi Y-H, Han Y-E, Oh S-J, Lee A, Lee B, Magnan R, Ryu SY, Choi CW, Kim MS. Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model. Antioxidants. 2021; 10(6):835. https://doi.org/10.3390/antiox10060835
Chicago/Turabian StyleHong, Yuni, Yun-Hyeok Choi, Young-Eun Han, Soo-Jin Oh, Ansoo Lee, Bonggi Lee, Rebecca Magnan, Shi Yong Ryu, Chun Whan Choi, and Min Soo Kim. 2021. "Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model" Antioxidants 10, no. 6: 835. https://doi.org/10.3390/antiox10060835