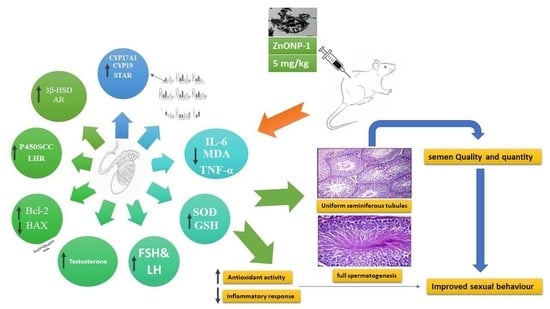
Insight Study on the Comparison between Zinc Oxide Nanoparticles and Its Bulk Impact on Reproductive Performance, Antioxidant Levels, Gene Expression, and Histopathology of Testes in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization of ZnONP
2.3. Animals and Experimental Procedures
2.4. Sexual Behavior (Fertility Test)
2.5. Pup’s Performance
2.6. Semen Characteristics
- Sperm motility: By cutting the cauda epididymis in a sterile petri dish, sperm were collected to allow the sperm to bathe out of the epididymal tubules. There was a drop of sperm suspension on a slide and a coverslip on it. Ten fields were examined at ×400 magnification by a phase-contrast microscope. The % of motile sperm from the total sperms counted was evaluated within 2−4 min.
- Sperm viability: A sperm suspension drop was mixed with one drop of 1% eosin Y/5% nigrosine, and a smear was made. Upon 2 min of incubation at room temperature, the slides were examined with magnification by a bright-field microscope at ×400. Per sample we counted one hundred sperms and the % viability was measured. Dead sperm were pink and live sperm were not stained.
- Sperm abnormalities: A drop of 1% eosin Y/5% nigrosine was put into one drop of the sperm suspension. Sperm smears were pulled out on clean, grate-free slides, and a hundred sperm were counted at ×400 for morphological abnormalities, such as amorphous, bicephalic, spiral, or irregular tails.
- Epididymal sperm count: Five μL of epididymal sperm suspension was diluted by 95 μL of solution (5 g NaCl and five drops of formalin/100 mL distilled water). One drop of the diluted epididymal content was moved to each of the hemocytometer counting chambers and allowed to dry for 5 min. During this period, the cells were deposited and numbered at ×400.
2.7. Reproductive Hormones Assay in Testes
2.8. Testicular Oxidative Markers
2.9. Real-Time Polymerase Chain Reaction (RT-PCR)
2.10. Histopathology
2.11. Immunohistochemistry and Quantitative Analysis
2.12. Data Analysis
3. Results
3.1. Characterization of the ZnONPs
3.2. Sexual Behavior
3.3. Semen Characteristics
3.4. Pup’s Performance
3.5. Reproductive Hormones
3.6. Testicular Oxidative Markers
3.7. Gene Expression Findings
3.8. Histopathological Findings
3.9. Immunohistochemistry and Quantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Consent to Participate
Conflicts of Interest
References
- Faddah, L.M.; Abdel Baky, N.A.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Fatani, A.J.; Atteya, M. Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2012, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free. Radic. Res. 2009, 44, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo e Sa, M.; Pezzato, L.E.; Barros, M.M.; De Magalhaes Padilha, P. Relative bioavailability of zinc in supplemental inorganic and organic sources for Nile tilapia Oreochromis niloticus fingerlings. Aquac. Nutr. 2005, 11, 273–281. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Ebisch, I.; Thomas, C.; Peters, W.; Braat, D.; Steegers-Theunissen, R. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Updat. 2007, 13, 163–174. [Google Scholar] [CrossRef]
- Bichão, H.; Borg-Karlson, A.-K.; Araújo, J.; Mustaparta, H. Five types of olfactory receptor neurons in the strawberry blossom weevil Anthonomus rubi: Selective responses to inducible host-plant volatiles. Chem. Senses 2005, 30, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Turgut, G.; Abban, G.; Turgut, S.; Take, G. Effect of overdose zinc on mouse testis and its relation with sperm count and motility. Biol. Trace Element Res. 2003, 96, 271–280. [Google Scholar] [CrossRef]
- Guan, R.; Kang, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602. [Google Scholar] [CrossRef] [Green Version]
- Alimohammadi, S.; Hosseini, M.S.; Behbood, L. Prenatal exposure to zinc oxide nanoparticles can induce depressive-like behaviors in mice offspring. Int. J. Pept. Res. Ther. 2018, 25, 401–409. [Google Scholar] [CrossRef]
- Chen, B.; Hong, W.; Yang, P.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Nano Zinc Oxide Induced Fetal Mice Growth Restriction, Based on Oxide Stress and Endoplasmic Reticulum Stress. Nanomaterials 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.; Jia, J.; Wang, Z.; Sharma, V.K.; Yan, B. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol. Environ. Saf. 2019, 182, 109439. [Google Scholar] [CrossRef] [PubMed]
- Mesallam, D.; Deraz, R.H.; Aal, S.M.A.; Ahmed, S.M. Toxicity of Subacute Oral Zinc Oxide Nanoparticles on Testes and Prostate of Adult Albino Rats and Role of Recovery. J. Histol. Histopathol. 2019, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Torabi, M.; Kesmati, M.; Harooni, H.; Varzi, H. Different efficacy of nanoparticle and conventional ZnO in an animal model of anxiety. Neurophysiology 2013, 45, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Tso, E.-F.; Tam, W. The effect of continuous treatment with prostaglandin F-2α on oestrous cycle length and corpus luteum regression in hysterectomized guinea-pigs. Reproduction 1977, 50, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Yakubu, M.; Akanji, M.; Oladiji, A. Male sexual dysfunction and methods used in assessing medicinal plants with aphrodisiac potentials. Pharmacogn. Rev. 2007, 1, 49–56. [Google Scholar]
- Luna, L. Berg’s method for spermatozoa. In Manual od Histological Staining Methods of the Armed Forces Institute of Pathology; Blakiston Division, McGraw-Hill: New York, NY, USA, 1968; pp. 117–118. [Google Scholar]
- Hamilton, H.; Lukefahr, S.; McNitt, J. Maternal nest quality and its influence on litter survival and weaning performance in commercial rabbits. J. Anim. Sci. 1997, 75, 926–933. [Google Scholar] [CrossRef]
- Rezvanfar, M.; Sadrkhanlou, R.; Ahmadi, A.; Shojaei-Sadee, H.; Rezvanfar, M.; Mohammadirad, A.; Salehnia, A.; Abdollahi, M. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum. Exp. Toxicol. 2008, 27, 901–910. [Google Scholar] [CrossRef]
- Demetrious, J.A. Testosterone in methods. In Clinical Chemistry, 2nd ed.; Tech, A.G., Kapalan, L.A., Eds.; Mosby: Los Angles, CA, USA, 1987; p. 268. [Google Scholar]
- Okaichi, Y.; Ishikura, Y.; Akimoto, K.; Kawashima, H.; Toyoda-Ono, Y.; Kiso, Y.; Okaichi, H. Arachidonic acid improves aged rats’ spatial cognition. Physiol. Behav. 2005, 84, 617–623. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Roa, N.; Yogi, K. Measurement of superoxide dismutase. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Horm. Res. Paediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.K.; Lucas, B.; Schrand, A.; Murdock, R.C.; Lee, T.; Schlager, J.J.; Hussain, S.M.; Hofmann, M.-C. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol. Sci. 2010, 116, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Ibraheem, S.R.; Ibrahim, M.R. Physiological and histological effects of (zinc and iron) oxide nanoparticles on some fertility parameters in female mice. Al-Mustansiriyah J. Sci. 2017, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moridian, M.; Khorsandi, L.; Talebi, A. Morphometric and stereological assessment of the effects of zinc oxide nanoparticles on the mouse testicular tissue. Bratisl Lek List. 2015, 116, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-H.; Ma, X.; Xu, Y.; Tang, H.; Yang, S.-T.; Yang, Y.-F.; Kang, D.-D.; Wang, H.; Liu, Y. Low toxicity and accumulation of zinc oxide nanoparticles in mice after 270-day consecutive dietary supplementation. Toxicol. Res. 2017, 6, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afifi, T.D. Divorce. In The International Encyclopedia of Interpersonal Communication; University of Iowa: Iowa City, IA, USA, 2015; pp. 1–7. [Google Scholar]
- Agarwal, A.; Said, T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update 2003, 9, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Strss on Male Reproduction. World J. Mens. Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; l’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, H.; Kayama, F.; Mori, N.; Doi, Y.; Fujimoto, S. Effects of dietary zinc deficiency on protein secretory functions of the mouse testis. Arch. Histol. Cytol. 1991, 54, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Küttler, K.; Deschl, U. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010, 38, 5S–81S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, L.; Min, L.-J.; Zhu, L.-Q.; Sun, Q.-Y.; Zhang, H.-F.; Liu, X.-Q.; Zhang, W.-D.; Ge, W.; Wang, J.-J. Regulation of MicroRNAs, and the correlations of MicroRNAs and their targeted genes by zinc oxide nanoparticles in ovarian granulosa cells. PLoS ONE 2016, 11, e0155865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.I.; Amira, F.A.; Manal, M.M.; Hanan, E.M. Effect of zinc oxide nanoparticles on the structure of testis of adult albino rats and the possible protective role of naringenin. Med. J. Cairo Univ. 2019, 87, 3469–3483. [Google Scholar] [CrossRef]
- Radhi, M.J.; Al-Bairuty, G.A.A.L. Effect of Zinc oxide nanoparticles (ZnO-NPs) on weights of some reproductive organs and sperm abnormalities in the tail of epididymis of albino mice. J. Pharm. Sci. Res. 2019, 11, 243–246. [Google Scholar]
- Badkoobeh, P.; Parivar, K.; Kalantar, S.M.; Hosseini, S.D.; Salabat, A. Effect of nano-zinc oxide on doxorubicin-induced oxidative stress and sperm disorders in adult male Wistar rats. Iran. J. Reprod. Med. 2013, 11, 355. [Google Scholar]
- Wang, B.; Feng, W.; Wang, M.; Wang, T.; Gu, Y.; Zhu, M.; Ouyang, H.; Shi, J.; Zhang, F.; Zhao, Y. Acute toxicological impact of nano-and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanopart. Res. 2008, 10, 263–276. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D. Biopersistence of silver nanoparticles in tissues from Sprague–Dawley rats. Part. Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Croxford, T.P.; McCormick, N.H.; Kelleher, S.L. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J. Nutr. 2011, 141, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Lim, J.-H.; Park, G.-J.; Maeng, E.-H.; Shin, J.-H.; Kim, Y.-R.; Kim, M.-K.; Lee, J.-K. Effect of zinc oxide nanoparticles on dams and embryo–fetal development in rats. Int. J. Nanomed. 2014, 9, 145. [Google Scholar]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.; Zhao, Y. Walking the line: The fate of nanomaterials at biological barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, J.; Loeschner, K.; Correia, M.; Larsen, E.H.; Manser, P.; Wichser, A.; Boodhia, K.; Al-Ahmady, Z.S.; Ruiz, J.; Astruc, D. Translocation of silver nanoparticles in the ex vivo human placenta perfusion model characterized by single particle ICP-MS. Nanoscale 2018, 10, 11980–11991. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Jo, E.; Seo, G.; Kwon, J.-T.; Lee, M.; cheun Lee, B.; Eom, I.; Kim, P.; Choi, K. Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J. Toxicol. Sci. 2013, 38, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Lim, J.-H.; Park, G.-J.; Maeng, E.-H.; Shin, J.-H.; Kim, M.-K.; Jeong, J.; Park, J.-A. Prenatal development toxicity study of zinc oxide nanoparticles in rats. Int. J. Nanomed. 2014, 9, 159. [Google Scholar]
- Abdella, A.; Elabed, B.; Bakhiet, A.; Gadir, W.; Adam, S. In vivo study on Lead, Cadmium and Zn supplementatitions on spermatogenesis in albino rats. J. Pharmacol. Toxicol. 2011, 6, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Sahin, K.; Kucuk, O. Zinc supplementation alleviates heat stress in laying Japanese quail. J. Nutr. 2003, 133, 2808–2811. [Google Scholar] [CrossRef]
- Hambidge, K. Trace elements in human and animal nutrition. Zinc 1986, 2, 13–19. [Google Scholar]
- Nazem, H.; Arefian, Z. Effect of ZnO NPs on Tumor Marker Hormones in Male Rats. Biomed. Res. 2015, 26, 82–88. [Google Scholar]
- Mozaffari, Z.; Parivar, K.; Roodbari, N.H.; Irani, S. The Impact of Zinc Oxide Nanoparticle on LH, FSH, and Testosterone Hormones in Mature Male NMRI Rats. J. Pharm. Res. Int. 2020, 30–37. [Google Scholar] [CrossRef]
- McAuliffe, M.E.; Perry, M.J. Are nanoparticles potential male reproductive toxicants? A literature review. Nanotoxicology 2007, 1, 204–210. [Google Scholar] [CrossRef]
- Metwaly, M.E. Ameliorative Effect of Zinc Oxide Nanoparticles on Antioxidants and Sperm Characteristics in Streptozotocin-Induced Diabetic Rat Testes. BioMed Res. Int. 2015, 2015, 153573. [Google Scholar]
- Afifi, M.; Abdelazim, A.M. Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Kvist, U. Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol. Scand. 1980, 109, 79–84. [Google Scholar] [CrossRef]
- Komatsu, T.; Tabata, M.; Kubo-Irie, M.; Shimizu, T.; Suzuki, K.-i.; Nihei, Y.; Takeda, K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol. Vitr. 2008, 22, 1825–1831. [Google Scholar] [CrossRef]
- Stocco, D. The role of the StAR protein in steroidogenesis: Challenges for the future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Ettlin, R.A.; Hikim, A.P.S.; Clegg, E.D. Histological and histopathological evaluation of the testis. Int. J. Androl. 1993, 16, 83. [Google Scholar] [CrossRef]
- Kumar, S.G.; Narayana, K.; Bairy, K.; D’Souza, U.J.; Samuel, V.P.; Gopalakrishna, K. Dacarbazine induces genotoxic and cytotoxic germ cell damage with concomitant decrease in testosterone and increase in lactate dehydrogenase concentration in the testis. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 2006, 607, 240–252. [Google Scholar] [CrossRef] [PubMed]










| Gene | Direction | Primer Sequence | Accession Number |
|---|---|---|---|
| Bax | Sense | GGCGAATTGGCGATGAACTG | NM_017059.2 |
| Antisense | ATGGTTCTGATCAGCTCGGG | ||
| Bcl-2 | Sense | GATTGTGGCCTTCTTTGAGT | NM_016993.1 |
| Antisense | ATAGTTCCACAAAGGCATCC | ||
| CYP17A1 | Sense | ACTGAGGGTATCGTGGATGC | NM_012753.2 |
| Antisense | TCGAACTTCTCCCTGCACTT | ||
| StAR | Sense | CTGCTAGACCAGCCCATGGAC | NM_031558.3 |
| Antisense | TGATTTCCTTGACATTTGGGTTCC | ||
| Cyp11a1 | Sense | AGGTGTAGCTCAGGACTT | J05156 |
| Antisense | AGGAGGCTATAAAGGACACC | ||
| 3β-HSD | Sense | CCCATACAGCAAAAGGATGG | M38178 |
| Antisense | GCCGCAAGTATCATGACAGA | ||
| Cyp19 | Sense | GCTTCTCATCGCAGAGTATCCGG | M33986 |
| Antisense | CAAGGGTAAATTCATTGGGCTTGG | ||
| LHR | Sense | CATTCAATGGGACGACTCTA | NM_012978.1 |
| Antisense | GCCTGCAATTTGGTGGA | ||
| AR | Sense | TTTGGACAGTACCAGGGACC | NM_012502.1 |
| Antisense | CTTCTGTTTCCCTTCCGCAG | ||
| GAPDH | Sense | TCAAGAAGGTGGTGAAGCAG | NM_017008.4 |
| Antisense | AGGTGGAAGAATGGGAGTTG |
| Testes/Lesion | Incidence 1 and Severity 2 of Histopathological Lesions | |||||||
|---|---|---|---|---|---|---|---|---|
| ZnONP-2 | BZnO-2 | |||||||
| - | + | ++ | +++ | - | + | ++ | +++ | |
| Depletion of germinal cells | 3 | 2 | 1 | 0 | 2 | 1 | 2 | 1 |
| Hyalinization of the luminal contents | 4 | 1 | 1 | 0 | 2 | 1 | 2 | 1 |
| Vacuolation of germ cells and Sertoli cells | 3 | 1 | 2 | 0 | 2 | 2 | 2 | 0 |
| Sloughing of the germinal epithelium | 2 | 3 | 1 | 0 | 1 | 2 | 1 | 2 |
| Shrunken, buckled, disorganized | 2 | 2 | 2 | 0 | 2 | 3 | 1 | 0 |
| Interstitial edema | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 2 |
| Giant cell formation | 6 | 0 | 0 | 0 | 2 | 3 | 1 | 0 |
| Interstitial inflammatory cell infiltration | 6 | 0 | 0 | 0 | 2 | 4 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goma, A.A.; Tohamy, H.G.; El-Kazaz, S.E.; Soliman, M.M.; Shukry, M.; Elgazzar, A.M.; Rashed, R.R. Insight Study on the Comparison between Zinc Oxide Nanoparticles and Its Bulk Impact on Reproductive Performance, Antioxidant Levels, Gene Expression, and Histopathology of Testes in Male Rats. Antioxidants 2021, 10, 41. https://doi.org/10.3390/antiox10010041
Goma AA, Tohamy HG, El-Kazaz SE, Soliman MM, Shukry M, Elgazzar AM, Rashed RR. Insight Study on the Comparison between Zinc Oxide Nanoparticles and Its Bulk Impact on Reproductive Performance, Antioxidant Levels, Gene Expression, and Histopathology of Testes in Male Rats. Antioxidants. 2021; 10(1):41. https://doi.org/10.3390/antiox10010041
Chicago/Turabian StyleGoma, Amira A., Hossam G. Tohamy, Sara E. El-Kazaz, Mohamed M. Soliman, Mustafa Shukry, Ahmed M. Elgazzar, and Rashed R. Rashed. 2021. "Insight Study on the Comparison between Zinc Oxide Nanoparticles and Its Bulk Impact on Reproductive Performance, Antioxidant Levels, Gene Expression, and Histopathology of Testes in Male Rats" Antioxidants 10, no. 1: 41. https://doi.org/10.3390/antiox10010041