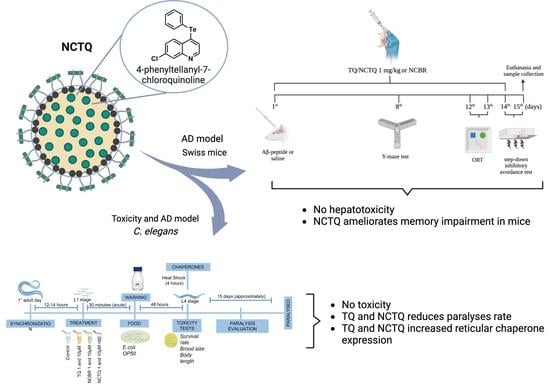
Development and In Vivo Assessment of 4-Phenyltellanyl-7-chloroquinoline-loaded Polymeric Nanocapsules in Alzheimer’s Disease Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Nanocapsules Preparation
2.3. Physicochemical Characterization of the Formulations
2.3.1. Particle Size, Zeta Potential, and pH
2.3.2. Drug Content Determination and Encapsulation Efficiency
2.3.3. HPLC-PDA Apparatus and Chromatographic Conditions
2.3.4. Method Validation and Sample Preparation
Linearity and Sensitivity
Precision
Accuracy
Robustness
Specificity
2.4. Alzheimer’s Disease Model in C. elegans
2.4.1. C. elegans Maintenance and Treatment
2.4.2. Toxicity Endpoints in C. elegans
2.4.3. Chaperones HSP-4 and HSP-6 Expression
2.4.4. Aβ Peptide Aggregation Model in C. elegans
2.5. Alzheimer’s Disease Model in Mice
2.5.1. Animals
2.5.2. Experimental Protocol
2.5.3. Behavioral Tests
Y-Maze Task
Object Recognition Task
Step-Down Inhibitory Avoidance
2.6. Ex-Vivo Assays
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of Nanocapsules
3.2. Validation of the Analytical Methodology
3.3. C. elegans Assays
3.3.1. NCTQ Did Not Elicit Toxicity in C. elegans
3.3.2. TQ and NCTQ Increased Reticular Chaperone (HSP-4) Expression
3.3.3. Treatment Reduced Aβ Peptide-Induced Paralysis Rate
3.4. Mice Assays
3.4.1. NCTQ Ameliorates Memory Impairment in Mice
Number of Arm Entries and Spontaneous Alternation Behavior
Object Recognition Task
Step-Down Inhibitory Avoidance Task
3.4.2. Effects of Treatments or Aβ Peptide in Ex Vivo Analyses in Mice: Treatments Did Not Become Hepatotoxic at the Tested Dose in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A Systemic View of Alzheimer Disease—Insights from Amyloid-β Metabolism beyond the Brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Pinz, M.P.; de Oliveira, R.L.; da Fonseca, C.A.R.; Voss, G.T.; da Silva, B.P.; Duarte, L.F.B.; Domingues, W.B.; Ortiz, H.G.; Savall, A.S.P.; Meotti, F.C.; et al. A Purine Derivative Containing an Organoselenium Group Protects against Memory Impairment, Sensitivity to Nociception, Oxidative Damage, and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1214–1231. [Google Scholar] [CrossRef]
- Martini, F.; Rosa, S.G.; Klann, I.P.; Fulco, B.C.W.; Carvalho, F.B.; Rahmeier, F.L.; Fernandes, M.C.; Nogueira, C.W. A Multifunctional Compound Ebselen Reverses Memory Impairment, Apoptosis and Oxidative Stress in a Mouse Model of Sporadic Alzheimer’s Disease. J. Psychiatr. Res. 2019, 109, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Pinton, S.; Souza, A.C.; Sari, M.H.M.; Ramalho, R.M.; Rodriguesb, C.M.P.; Nogueiraa, C.W. P,P′-Methoxyl-Diphenyl Diselenide Protects against Amyloid-β Induced Cytotoxicity in Vitro and Improves Memory Deficits in Vivo. Behav. Brain Res. 2013, 247, 241–247. [Google Scholar] [CrossRef]
- Giordani, C.F.A.; De Souza, D.; Dornelles, L.; Nogueira, C.W.; Alves, M.P.; Prigol, M.; Rodrigues, O.E.D. Diphenyl Diselenide-Loaded Nanocapsules: Preparation and Biological Distribution. Appl. Biochem. Biotechnol. 2014, 172, 755–766. [Google Scholar] [CrossRef]
- Marcondes Sari, M.H.; Zborowski, V.A.; Ferreira, L.M.; Jardim, N.D.S.; Araujo, P.C.O.; Brüning, C.A.; Cruz, L.; Nogueira, C.W. Enhanced Pharmacological Actions of p,p’-Methoxyl-Diphenyl Diselenide-Loaded Polymeric Nanocapsules in a Mouse Model of Neuropathic Pain: Behavioral and Molecular Insights. J. Trace Elem. Med. Biol. 2018, 46, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Cervi, V.F.; Sari, M.H.M.; Barbieri, A.V.; Ramos, A.P.; Copetti, P.M.; de Brum, G.F.; Nascimento, K.; Nadal, J.M.; Farago, P.V.; et al. Diphenyl Diselenide Loaded Poly(ε-Caprolactone) Nanocapsules with Selective Antimelanoma Activity: Development and Cytotoxic Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 1–9. [Google Scholar] [CrossRef]
- Marcondes Sari, M.H.; Ferreira, L.M.; Zborowski, V.A.; Araujo, P.C.O.; Cervi, V.F.; Brüning, C.A.; Cruz, L.; Nogueira, C.W. P,p’-Methoxyl-Diphenyl Diselenide-Loaded Polymeric Nanocapsules Are Chemically Stable and Do Not Induce Toxicity in Mice. Eur. J. Pharm. Biopharm. 2017, 117, 39–48. [Google Scholar] [CrossRef]
- Pinz, M.P.; dos Reis, A.S.; Vogt, A.G.; Krüger, R.; Alves, D.; Jesse, C.R.; Roman, S.S.; Soares, M.P.; Wilhelm, E.A.; Luchese, C. Current Advances of Pharmacological Properties of 7-Chloro-4-(Phenylselanyl) Quinoline: Prevention of Cognitive Deficit and Anxiety in Alzheimer’s Disease Model. Biomed. Pharmacother. 2018, 105, 1006–1014. [Google Scholar] [CrossRef]
- Pinz, M.; Reis, A.S.; Duarte, V.; Da Rocha, M.J.; Goldani, B.S.; Alves, D.; Savegnago, L.; Luchese, C.; Wilhelm, E.A. 4-Phenylselenyl-7-Chloroquinoline, a New Quinoline Derivative Containing Selenium, Has Potential Antinociceptive and Anti-Inflammatory Actions. Eur. J. Pharmacol. 2016, 780, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.; Barth, A.; da Costa, G.P.; Alves, D.; Novo, D.L.R.; Mesko, M.F.; Wilhelm, E.A. Role of 7-Chloro-4-(Phenylselanyl) Quinoline as an Anti-Aging Drug Fighting Oxidative Damage in Different Tissues of Aged Rats. Exp. Gerontol. 2020, 130, 110804. [Google Scholar] [CrossRef]
- Souza, A.C.G.; Sari, M.H.M.; Pinton, S.; Luchese, C.; Neto, J.S.S.; Nogueira, C.W. 2-Phenylethynyl-Butyltellurium Attenuates Amyloid-β Peptide(25-35)-Induced Learning and Memory Impairments in Mice. J. Neurosci. Res. 2013, 91, 848–853. [Google Scholar] [CrossRef]
- Salgueiro, W.G.; Goldani, B.S.; Peres, T.V.; Miranda-Vizuete, A.; Aschner, M.; da Rocha, J.B.T.; Alves, D.; Ávila, D.S. Insights into the Differential Toxicological and Antioxidant Effects of 4-Phenylchalcogenil-7-Chloroquinolines in Caenorhabditis Elegans. Free Radic. Biol. Med. 2017, 110, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ávila, D.S.; Colle, D.; Gubert, P.; Palma, A.S.; Puntel, G.; Manarin, F.; Noremberg, S.; Nascimento, P.C.; Aschner, M.; Rocha, J.B.T.; et al. A Possible Neuroprotective Action of a Vinylic Telluride against Mn-Induced Neurotoxicity. Toxicol. Sci. 2010, 115, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, D.S.; Benedetto, A.; Au, C.; Manarin, F.; Erikson, K.; Soares, F.A.; Rocha, J.B.T.; Aschner, M. Organotellurium and Organoselenium Compounds Attenuate Mn-Induced Toxicity in Caenorhabditis Elegans by Preventing Oxidative Stress. Free Radic. Biol. Med. 2012, 52, 1903–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Machado, N.C.; Pedroso, A.B.; Goldani, B.S.; Seus, N.; Moura, S.; Savegnago, L.; Jacob, R.G.; Alves, D. Organocatalytic Synthesis and Evaluation of 7-Chloroquinoline-1,2,3-Triazoyl Carboxamides as Potential Antinociceptive, Anti-Inflammatory and Anticonvulsant Agent. RSC Adv. 2014, 40, 41437–41445. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; De Paula Oliveira, R. Guarana ( Paullinia Cupana) Extract Protects Caenorhabditis Elegans Models for Alzheimer Disease and Huntington Disease through Activation of Antioxidant and Protein Degradation Pathways. Oxid. Med. Cell Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Zhao, W.; Ho, L.; Wang, J.; Walsh, K.; Gandy, S.; Pasinetti, G.M. Regulation of Forkhead Transcription Factor FoxO3a Contributes to Calorie Restriction-Induced Prevention of Alzheimer’s Disease-Type Amyloid Neuropathology and Spatial Memory Deterioration. Ann. N. Y. Acad. Sci. 2008, 1147, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Bahn, G.; Jo, D.G. Therapeutic Approaches to Alzheimer’s Disease Through Modulation of NRF2. Neuromol. Med. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Nakama, K.A.; Pacheco, C.O.; de Gomes, M.G.; de Souza, J.F.; de Souza Pinto, A.C.; de Oliveira, F.A.; da Fonseca, A.L.; Varotti, F.; Fajardo, A.R.; et al. Curcumin-Loaded Nanocapsules: Influence of Surface Characteristics on Technological Parameters and Potential Antimalarial Activity. Mat. Sci. Eng.C 2021, 118, 111356. [Google Scholar] [CrossRef]
- Vieira, S.M.; Michels, L.R.; Roversi, K.; Metz, V.G.; Moraes, B.K.S.; Piegas, E.M.; Freddo, R.J.; Gundel, A.; Costa, T.D.; Burger, M.E.; et al. A Surface Modification of Clozapine-Loaded Nanocapsules Improves Their Efficacy: A Study of Formulation Development and Biological Assessment. Colloids Surf. B Biointerfaces 2016, 145, 748–756. [Google Scholar] [CrossRef]
- da Costa Güllich, A.A.; Coelho, R.P.; Pilar, B.C.; Ströher, D.J.; Galarça, L.A.S.L.; Vieira, S.M.; da Costa Escobar Piccoli, J.; Haas, S.E.; Manfredini, V. Clozapine Linked to Nanocapsules Minimizes Tissue and Oxidative Damage to Biomolecules Lipids, Proteins and DNA in Brain of Rats Wistar. Metab. Brain Dis. 2015, 30, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Velasques, K.; Maciel, T.R.; Forno, A.H.D.C.D.; Teixeira, F.E.G.; da Fonseca, A.L.; Varotti, F.D.P.; Fajardo, A.R.; Avila, D.; Haas, S.E. Co-Nanoencapsulation of Antimalarial Drugs Increases Their in Vitro Efficacy against Plasmodium Falciparum and Decreases Their Toxicity to Caenorhabditis Elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.B.; Funguetto-Ribeiro, A.C.; Maciel, T.R.; Fonseca, D.P.; Favarin, F.R.; Nogueira-Librelotto, D.R.; de Gomes, M.G.; Nakamura, T.U.; Rolim, C.M.B.; Haas, S.E. In Vivo and in Vitro per Se Effect Evaluation of Polycaprolactone and Eudragit® RS100-Based Nanoparticles. Biomed. Pharmacother. 2022, 153, 113410. [Google Scholar] [CrossRef]
- Ianiski, F.R.; Alves, C.B.; Souza, A.C.G.; Pinton, S.; Roman, S.S.; Rhoden, C.R.B.; Alves, M.P.; Luchese, C. Protective Effect of Meloxicam-Loaded Nanocapsules against Amyloid-β Peptide-Induced Damage in Mice. Behav. Brain Res. 2012, 230, 100–107. [Google Scholar] [CrossRef]
- Giacomeli, R.; Izoton, J.C.; dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective Effects of Curcumin Lipid-Core Nanocapsules in a Model Alzheimer’s Disease Induced by β-Amyloid 1-42 Peptide in Aged Female Mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.Z.; Savall, A.S.P.; Da Luz Abreu, E.; Nakama, K.A.; Dos Santos, R.B.; Guedes, M.C.M.; Ávila, D.S.; Luchese, C.; Haas, S.E.; Quines, C.B.; et al. Co-Nanoencapsulated Meloxicam and Curcumin Improves Cognitive Impairment Induced by Amyloid-Beta through Modulation of Cyclooxygenase-2 in Mice. Neural. Regen. Res. 2021, 16, 783–789. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Pacheco, C.; de Gomes, M.G.; da Silva Neto, M.R.; Parisotto, A.J.M.; dos Santos, R.B.; Maciel, T.R.; Ribeiro, A.C.F.; Giacomeli, R.; Haas, S.E. Surface-Functionalized Curcumin-Loaded Polymeric Nanocapsules Could Block Apomorphine-Induced Behavioral Changes in Rats. Pharmacol. Rep. 2022, 74, 135–147. [Google Scholar] [CrossRef]
- Savegnago, L.; Vieira, A.I.; Seus, N.; Goldani, B.S.; Castro, M.R.; Lenardão, E.J.; Alves, D. Synthesis and Antioxidant Properties of Novel Quinoline-Chalcogenium Compounds. Tetra. Lett. 2013, 54, 40–44. [Google Scholar] [CrossRef] [Green Version]
- ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2-r1-validation-analytical-procedures-text-and-methodology (accessed on 12 December 2020).
- FDA. Center for Drug Evaluation and Research (CDER)—Reviewer Guidance’ Validation of Chromatographic Methods; Center for Drug Evaluation and Research: Washington, DC, USA, 1994. [Google Scholar]
- ANVISA RESOLUÇÃO RDC No 166, DE 24 DE JULHO DE 2017—Dispõe Sobre a Validação de Métodos Analíticose e Dá Outras Providências. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/19194581/do1-2017-07-25-resolucao-rdc-n-166-de-24-de-julho-de-2017-19194412 (accessed on 12 December 2020).
- Zevian, S.C.; Yanowitz, J.L. Methodological Considerations for Heat Shock of the Nematode Caenorhabditis Elegans. Methods 2014, 68, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.S.D.O.; Barbosa, F.A.R.; Canto, R.F.S.; Lucchese, C.; Pinton, S.; Braga, A.L.; de Azeredo, J.B.; Quines, C.B.; Ávila, D.S. Dihydropyrimidinone-Derived Selenoesters Efficacy and Safety in an in Vivo Model of Aβ Aggregation. Neurotoxicology 2022, 88, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Reis, N.A.P.; de Oliveira Pereira, F.S.; Ávila, D.S.; Guerra, C.C.; Flores da Silva, L.; Junges, C.H.; Ferrão, M.F.; Bergold, A.M. Grape Juice Reduces the Effects of Amyloid β Aggregation Phenotype and Extends the Longevity in Caenorhabditis Elegans. Nutr. Neurosci. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 1996. [Google Scholar]
- Haley, T.J.; Mccormik, W.G. Pharmacological Effects Produced by Intracerebral Injection of Drugs in the Conscious Mouse. Br. J. Pharmacol. Chemother. 1957, 12, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Sarter, M.; Bodewitz, G.; Stephens, D.N. Attenuation of Scopolamine-Induced Impairment of Spontaneous Alternation Behaviour by Antagonist but Not Inverse Agonist and Agonist β-Carbolines. Psychopharmacology 1988, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, E.C.; Rocha, J.B.T.; Nogueira, C.W. Diphenyl Ditelluride Impairs Short-Term Memory and Alters Neurochemical Parameters in Young Rats. Pharmacol. Biochem. Behav. 2009, 91, 430–435. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Koseki, M.; Wakamatsu, M.; Matsumura, E. Effects of Systemic Administration of β-Casomorphin-5 on Learning and Memory in Mice. Eur. J. Pharmacol. 2006, 530, 81–87. [Google Scholar] [CrossRef]
- Link, C.D. Expression of Human β-Amyloid Peptide in Transgenic Caenorhabditis Elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef] [Green Version]
- Dimer, F.A.; Pigatto, M.C.; Boque, C.A.; Pase, C.S.; Roversi, K.; Pohlmann, A.R.; Burger, M.E.; Rates, S.M.K.; Costa, T.D.; Guterres, S.S. Nanoencapsulation Improves Relative Bioavailability and Antipsychotic Effect of Olanzapine in Rats. J. Biomed. Nanotechnol. 2014, 11, 1482–1493. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R. V Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Moraes, B.K.S.; Vieira, S.M.; Salgueiro, W.G.; Michels, L.R.; Colomé, L.M.; Avila, D.S.; Haas, S.E. Clozapine-Loaded Polysorbate-Coated Polymeric Nanocapsules: Physico-Chemical Characterization and Toxicity Evaluation in Caenorhabditis Elegans Model. J. Nanosci. Nanotechnol. 2016, 16, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Nakama, K.A.; dos Santos, R.B.; da Rosa Silva, C.E.; Izoton, J.C.; Savall, A.S.P.; Gutirrez, M.E.Z.; Roman, S.S.; Luchese, C.; Pinton, S.; Haas, S.E. Establishment of Analytical Method for Quantification of Anti-Inflammatory Agents Co-Nanoencapsulated and Its Application to Physicochemical Development and Characterization of Lipid-Core Nanocapsules. Arab. J. Chem. 2020, 13, 2456–2469. [Google Scholar] [CrossRef]
- Guan, W.; Ma, Y.; Ding, S.; Liu, Y.; Song, Z.; Liu, X.; Tang, L.; Wang, Y. The Technology for Improving Stability of Nanosuspensions in Drug Delivery. J. Nano Res. 2022, 24, 14. [Google Scholar] [CrossRef]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent Effect of Polymeric Nanoparticle Zeta Potential/Surface Charge, on Their Cytotoxicity and Affinity to Cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef]
- Maynard, A.D.; Warheit, D.B.; Philbert, M.A. The New Toxicology of Sophisticated Materials: Nanotoxicology and Beyond. Toxicol. Sci. 2011, 120, S109–S129. [Google Scholar] [CrossRef]
- Alexander, A.G.; Marfil, V.; Li, C. Use of C. Elegans as a Model to Study Alzheimer’s Disease and Other Neurodegenerative Diseases. Front. Genet. 2014, 5, 279. [Google Scholar] [CrossRef] [Green Version]
- Shaye, D.D.; Greenwald, I. Ortholist: A Compendium of C. Elegans Genes with Human Orthologs. PLoS ONE 2011, 6, e20085. [Google Scholar] [CrossRef]
- Cohen, E.; Bieschke, J.; Perciavalle, R.M.; Kelly, J.W.; Dillin, A. Opposing Activities Protect against Age-Onset Proteotoxicity. Science 2006, 313, 1604–1610. [Google Scholar] [CrossRef]
- Martín-Peña, A.; Rincón-Limas, D.E.; Fernandez-Fúnez, P. Engineered Hsp70 Chaperones Prevent Aβ42-Induced Memory Impairments in a Drosophila Model of Alzheimer’s Disease. Sci. Rep. 2018, 8, 9915. [Google Scholar] [CrossRef] [Green Version]
- Govindan, J.A.; Jayamani, E.; Ruvkun, G. ROS-Based Lethality of Caenorhabditis Elegans Mitochondrial Electron Transport Mutants Grown on Escherichia Coli Siderophore Iron Release Mutants. Proc. Natl. Acad. Sci. USA 2019, 116, 21651–21658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, M.E.; Marini, S.; Coletta, M.; Orsini, F.; Giardina, B.; Misiti, F. Aβ(31-35) and Aβ(25-35) Fragments of Amyloid Beta-Protein Induce Cellular Death through Apoptotic Signals: Role of the Redox State of Methionine-35. FEBS Lett. 2005, 579, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Okonkwo, O.C.; Oishi, K.; Mori, S.; Tighe, S.; Miller, M.I.; Ceritoglu, C.; Brown, T.; Albert, M.; Lyketsos, C.G. Fornix Integrity and Hippocampal Volume Predict Memory Decline and Progression to Alzheimer’s Disease. Alzheimer’s Dement. 2012, 8, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benninghoff, J.; Perneczky, R. Anti-Dementia Medications and Anti-Alzheimer’s Disease Drugs: Side Effects, Contraindications, and Interactions. NeuroPsychoph 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Hirono, H.; Watanabe, K.; Hasegawa, K.; Hiroyasu, K.; Shibasaki, K.; Ohkoshi, S. Anti-Dementia Drugs and Hepatotoxicity–Report of Two Cases. Int. J. Gerontol. 2018, 12, 261–263. [Google Scholar] [CrossRef]








| NCTQ | NCBR | |
|---|---|---|
| D[4,3] (nm) | 240 ± 6 | 231 ± 6 |
| Span | 1.57 ± 0.04 | 1.58 ± 0.15 |
| Zeta potential (mV) | −25.37 ± 1.52 | −24.36 ± 5.54 |
| pH | 6.45 ± 0.07 | 6.29 ± 2.63 |
| EE (%) | 100 | - |
| Drug content (%) | 97.80 ± 0.37 | - |
| TQ | ||
|---|---|---|
| Mean (%) | RSD (%) | |
| Inter-day | 99.77 | 1.15 |
| Intra-day | 99.87 | 1.27 |
| Conditions | TQ | |||||
|---|---|---|---|---|---|---|
| Rt (min) | T (≤2.0) | K (≥2.0) | N (≥2000) | Mean (%) | RSD (%) | |
| Proposed method * | 8.20 | 1.7 | 4.5 | 8012 | 101.23 | 1 |
| 0.9 mL/min | 8.90 | 1.7 | 2.4 | 7570 | 110.3 | 6.8 |
| 1.1 mL/min | 7.20 | 1.7 | 2.4 | 7274 | 91.3 | 6.6 |
| pH (6.8) | 7.90 | 1.7 | 2.4 | 7338 | 100.5 | 0.33 |
| pH (7.2) | 7.80 | 1.7 | 2.4 | 7259 | 100.5 | 0.34 |
| Sham | TQ | NCTQ | Aβ | Aβ + TQ | Aβ + NCTQ | |
|---|---|---|---|---|---|---|
| AST(U/L) | 146.7 ± 5.2 | 168.6 ± 14.9 | 147.5 ± 10.2 | 157.5 ± 8.9 | 136.6 ± 9.1 | 174.9 ± 16.8 |
| ALT(U/L) | 35.2 ± 1.1 | 35.3 ± 5.7 | 45.1 ± 5.9 | 41.7 ± 5.6 | 39.7 ± 3.2 | 51.0 ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funguetto-Ribeiro, A.C.; Nakama, K.A.; Pinz, M.P.; Oliveira, R.L.d.; Sacramento, M.d.; Pereira, F.S.O.; Pinton, S.; Wilhelm, E.A.; Luchese, C.; Alves, D.; et al. Development and In Vivo Assessment of 4-Phenyltellanyl-7-chloroquinoline-loaded Polymeric Nanocapsules in Alzheimer’s Disease Models. Brain Sci. 2023, 13, 999. https://doi.org/10.3390/brainsci13070999
Funguetto-Ribeiro AC, Nakama KA, Pinz MP, Oliveira RLd, Sacramento Md, Pereira FSO, Pinton S, Wilhelm EA, Luchese C, Alves D, et al. Development and In Vivo Assessment of 4-Phenyltellanyl-7-chloroquinoline-loaded Polymeric Nanocapsules in Alzheimer’s Disease Models. Brain Sciences. 2023; 13(7):999. https://doi.org/10.3390/brainsci13070999
Chicago/Turabian StyleFunguetto-Ribeiro, Ana Cláudia, Kelly Ayumi Nakama, Mikaela Peglow Pinz, Renata Leivas de Oliveira, Manoela do Sacramento, Flávia S. Oliveira Pereira, Simone Pinton, Ethel Antunes Wilhelm, Cristiane Luchese, Diego Alves, and et al. 2023. "Development and In Vivo Assessment of 4-Phenyltellanyl-7-chloroquinoline-loaded Polymeric Nanocapsules in Alzheimer’s Disease Models" Brain Sciences 13, no. 7: 999. https://doi.org/10.3390/brainsci13070999