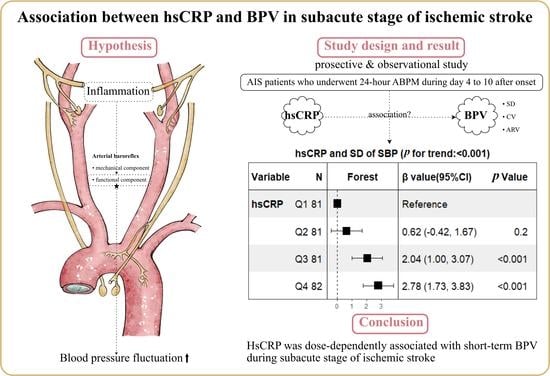
Association between High-Sensitivity C-Reactive Protein and Blood Pressure Variability in Subacute Stage of Ischemic Stroke
Abstract
:1. Introduction
2. Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Patients
2.3. Data Collection
2.3.1. General Assessment
2.3.2. hsCRP Assessment
2.3.3. Short-Term BPV Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qureshi, A.I. Acute hypertensive response in patients with stroke: Pathophysiology and management. Circulation 2008, 118, 176–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonoff, V.; Zhang, H.; Richart, T.; Staessen, J.A. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. 2009, 8, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cámara, R.; González-Bernal, J.; Aguilar-Parra, J.M.; Trigueros, R.; López-Liria, R.; González-Santos, J. Factors related to prehospital time in caring for patients with stroke. Emergencias 2021, 33, 454–463. [Google Scholar] [PubMed]
- Kang, J.; Ko, Y.; Park, J.H.; Kim, W.-J.; Jang, M.S.; Yang, M.H.; Lee, J.; Han, M.-K.; Gorelick, P.B.; Bae, H.-J. Effect of blood pressure on 3-month functional outcome in the subacute stage of ischemic stroke. Neurology 2012, 79, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kim, B.J.; Yang, M.H.; Jang, M.S.; Han, M.K.; Lee, J.S.; Gorelick, P.B.; Lee, J.; Bae, H.-J. Blood pressure variability in subacute stage and risk of major vascular events in ischemic stroke survivors. J. Hypertens. 2019, 37, 2000–2006. [Google Scholar] [CrossRef]
- Naito, H.; Hosomi, N.; Kuzume, D.; Nezu, T.; Aoki, S.; Morimoto, Y.; Kinboshi, M.; Yoshida, T.; Shiga, Y.; Kinoshita, N.; et al. Increased blood pressure variability during the subacute phase of ischemic stroke is associated with poor functional outcomes at 3 months. Sci. Rep. 2020, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Matsuo, R.; Kamouchi, M.; Kiyuna, F.; Sato, N.; Nakamura, K.; Hata, J.; Wakisaka, Y.; Ago, T.; Imaizumi, T.; et al. Day-by-Day Blood Pressure Variability in the Subacute Stage of Ischemic Stroke and Long-Term Recurrence. Stroke 2022, 53, 70–78. [Google Scholar] [CrossRef]
- Hawkes, M.A.; Anderson, C.S.; Rabinstein, A.A. Blood Pressure Variability After Cerebrovascular Events: A Possible New Therapeutic Target: A Narrative Review. Neurology 2022, 99, 150–160. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol. 2013, 10, 143–155. [Google Scholar] [CrossRef]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Cunningham, J.T.; Sullivan, M.J.; Wachtel, R.E.; Abboud, F.M. Structural versus functional modulation of the arterial baroreflex. Hypertension 1995, 26, 341–347. [Google Scholar] [CrossRef]
- Li, Z.; Mao, H.Z.; Abboud, F.M.; Chapleau, M.W. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ. Res. 1996, 79, 802–811. [Google Scholar] [CrossRef]
- Courties, G.; Herisson, F.; Sager, H.B.; Heidt, T.; Ye, Y.; Wei, Y.; Sun, Y.; Severe, N.; Dutta, P.; Scharff, J.; et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 2015, 116, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Simats, A.; Liesz, A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol. Med. 2022, 14, e16269. [Google Scholar] [CrossRef]
- Fukuda, K.; Kai, H.; Kamouchi, M.; Hata, J.; Ago, T.; Nakane, H.; Imaizumi, T.; Kitazono, T.; FSR Investigators; Steering Committee of the Fukuoka Stroke Registry Included. Day-by-Day Blood Pressure Variability and Functional Outcome After Acute Ischemic Stroke: Fukuoka Stroke Registry. Stroke 2015, 46, 1832–1839. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Fan, S.; Bian, Y.; Yang, Q.; Long, Z.; Chen, L.; Tang, W.; Zhang, N.; Zhen, Y.; Li, Z. Two-way comparison of brain perfusion image processing software for patients with acute ischemic strokes in real-world. Neuroradiology 2022, 64, 161–169. [Google Scholar] [CrossRef]
- Manning, L.; Hirakawa, Y.; Arima, H.; Wang, X.; Chalmers, J.; Wang, J.; Lindley, R.; Heeley, E.; Delcourt, C.; Neal, B.; et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: A post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014, 13, 364–373. [Google Scholar] [CrossRef]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpúrua, J.A.; Maestre, G.; Sulbarán, T. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens. 2005, 23, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.; Park, J.H.; Yang, M.H.; Ko, S.B.; Han, M.K.; Oh, C.W.; Lee, J.; Lee, J.; Bae, H.-J. The significance of blood pressure variability for the development of hemorrhagic transformation in acute ischemic stroke. Stroke 2010, 41, 2512–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Mederos, R.; Ribo, M.; Rovira, A.; Rubiera, M.; Munuera, J.; Santamarina, E.; Delgado, P.; Maisterra, O.; Alvarez-Sabin, J.; Molina, C.A. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology 2008, 71, 552–558. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, N.; Kang, J.; Park, S.H.; Kim, W.J.; Ko, Y.; Park, J.H.; Lee, J.S.; Lee, J.; Yang, M.H.; et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J. Hypertens. 2015, 33, 2099–2106. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Yang, P.; Zhu, Z.; Shi, M.; Peng, Y.; Zhong, C.; Wang, A.; Xu, T.; Peng, H.; et al. Blood Pressure Fluctuation During Hospitalization and Clinical Outcomes Within 3 Months After Ischemic Stroke. Hypertension 2022, 79, 2336–2345. [Google Scholar] [CrossRef]
- Aries, M.J.; Elting, J.W.; De Keyser, J.; Kremer, B.P.; Vroomen, P.C. Cerebral autoregulation in stroke: A review of transcranial Doppler studies. Stroke 2010, 41, 2697–2704. [Google Scholar] [CrossRef]
- Stead, L.G.; Gilmore, R.M.; Vedula, K.C.; Weaver, A.L.; Decker, W.W.; Brown, R.D., Jr. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology 2006, 66, 1878–1881. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Poulter, N.R.; Sever, P.S.; on behalf of the ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010, 9, 469–480. [Google Scholar] [CrossRef]
- Webb, A.J.; Fischer, U.; Mehta, Z.; Rothwell, P.M. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 2010, 375, 906–915. [Google Scholar] [CrossRef]
- Umemoto, S.; Ogihara, T.; Matsuzaki, M.; Rakugi, H.; Ohashi, Y.; Saruta, T. Effects of calcium channel blocker-based combinations on intra-individual blood pressure variability: Post hoc analysis of the COPE trial. Hypertens. Res. 2016, 39, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ladenvall, C.; Jood, K.; Blomstrand, C.; Nilsson, S.; Jern, C.; Ladenvall, P. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 2006, 37, 2018–2023. [Google Scholar] [CrossRef] [Green Version]
- Di Napoli, M.; Papa, F. Association between blood pressure and C-reactive protein levels in acute ischemic stroke. Hypertension 2003, 42, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Abramson, J.L.; Lewis, C.; Murrah, N.V.; Anderson, G.T.; Vaccarino, V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability in healthy adults. Am. J. Cardiol. 2006, 98, 649–651. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Lemmens, R.; Tsivgoulis, G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke 2021, 52, 2697–2706. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Xie, H.H.; Shu, H.; Yuan, W.J.; Su, D.F. Inflammation is involved in the organ damage induced by sinoaortic denervation in rats. J. Hypertens. 2003, 21, 2141–2148. [Google Scholar] [CrossRef]


| Characteristics | Total (n = 325) | hsCRP < 2 mg/L (n = 186) | hsCRP ≥ 2 mg/L (n = 139) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean ± SD | 60.0 ± 11.5 | 59.8 ± 10.5 | 60.1 ± 12.6 | 0.808 |
| Male, n (%) | 234 (72.0) | 135 (72.6) | 99 (71.2) | 0.787 |
| BMI, kg/m2, mean ± SD | 26.1 ± 3.6 | 25.9 ± 3.5 | 26.3 ± 3.7 | 0.224 |
| Smoking, n (%) | 150 (46.2) | 86 (46.2) | 64 (46.0) | 0.972 |
| Medical histories, n (%) | ||||
| Hypertension | 230 (70.8) | 127 (68.3) | 103 (74.1) | 0.254 |
| Diabetes mellitus | 113 (34.8) | 61 (32.8) | 52 (37.4) | 0.387 |
| Atrial fibrillation | 20 (6.2) | 12 (6.5) | 8 (5.8) | 0.796 |
| Coronary heart disease | 24 (7.4) | 11 (5.9) | 13 (9.4) | 0.241 |
| Prior TIA or stroke | 71 (21.8) | 38 (20.4) | 33 (23.7) | 0.475 |
| Clinical features | ||||
| Initial NIHSS, median (IQR) | 2 (1, 5) | 2 (1, 4) | 3 (1, 6) | 0.003 |
| Infarct volume, mL, median (IQR) | 1.9 (0.4, 22.8) | 1.2 (0.3, 15.6) | 2.5 (0.5, 34.8) | 0.001 |
| Onset to ABPM a, d, median (IQR) | 7 (6, 9) | 8 (6, 9) | 7 (6, 9) | 0.122 |
| Antihypertensive therapy b, n (%) | 92 (28.3) | 51 (27.4) | 41 (29.5) | 0.681 |
| WBC, ×10^9/L, mean ± SD | 6.9 ± 1.8 | 6.4 ± 1.6 | 7.5 ± 1.8 | <0.001 |
| NEUT, ×10^9/L, mean ± SD | 4.3 ± 1.5 | 3.9 ± 1.2 | 4.8 ± 1.5 | <0.001 |
| MONO, ×10^9/L, mean ± SD | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 | <0.001 |
| LYM, ×10^9/L, mean ± SD | 2.0 ± 0.7 | 1.9 ± 0.6 | 2 ± 0.7 | 0.089 |
| LDL, mmol/L, mean ± SD | 2.4 ± 0.8 | 2.3 ± 0.8 | 2.4 ± 0.8 | 0.459 |
| HDL, mmol/L, mean ± SD | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.870 |
| TOAST classification, n (%) | 0.216 | |||
| LAA | 162 (49.8) | 85 (45.7) | 77 (55.4) | |
| CE | 18 (5.5) | 12 (6.5) | 6 (4.3) | |
| SVO | 125 (38.5) | 80 (43) | 45 (32.4) | |
| OD | 5 (1.5) | 2 (1.1) | 3 (2.2) | |
| UD | 15 (4.6) | 7 (3.8) | 8 (5.8) | |
| Lesion patterns, n (%) | 0.033 | |||
| Anterior circulation | 202 (62.2) | 114 (61.3) | 88 (63.3) | |
| Posterior circulation | 92 (28.3) | 60 (32.3) | 32 (23) | |
| Anterior and posterior circulation | 31 (9.5) | 12 (6.5) | 19 (13.7) | |
| BP Parameters | Total (n = 325) | HsCRP < 2 mg/L (n = 186) | hsCRP ≥ 2 mg/L (n = 139) | p Value |
|---|---|---|---|---|
| SBPmean | 142.1 ± 16.9 | 138.2 ± 15.6 | 147.2 ± 17.3 | <0.001 |
| SBPSD | 13.8 ± 3.5 | 12.6 ± 3.0 | 15.2 ± 3.6 | <0.001 |
| SBPCV | 9.7 ± 2.3 | 9.2 ± 1.9 | 10.5 ± 2.5 | <0.001 |
| SBPARV | 12.8 ± 3.1 | 11.9 ± 2.7 | 14 ± 3.1 | <0.001 |
| DBPmean | 82.4 ± 10.9 | 81.8 ± 9.5 | 83.4 ± 12.5 | 0.184 |
| DBPSD | 9.9 ± 2.4 | 9.4 ± 2.3 | 10.5 ± 2.5 | <0.001 |
| DBPCV | 12.1 ± 3.1 | 11.6 ± 2.8 | 12.8 ± 3.3 | <0.001 |
| DBPARV | 8.9 ± 2.2 | 8.4 ± 2.0 | 9.5 ± 2.4 | <0.001 |
| Indices | Mean | SD | CV | ARV | ||||
|---|---|---|---|---|---|---|---|---|
| β Value (95% CI) | p Value | β Value (95% CI) | p Value | β Value (95% CI) | p Value | β Value (95% CI) | p Value | |
| SBP | ||||||||
| hsCRP ≥ 2 mg/L | 6.94 (3.39, 10.48) | <0.001 | 2.29 (1.55, 3.04) | <0.001 | 1.21 (0.72, 1.71) | <0.001 | 1.9 (1.24, 2.56) | <0.001 |
| DBP | ||||||||
| hsCRP ≥ 2 mg/L | 0.99 (−1.29, 3.27) | 0.392 | 0.93 (0.41, 1.46) | <0.001 | 1.12 (0.44, 1.8) | 0.001 | 0.93 (0.45, 1.41) | <0.001 |
| Subgroup | Mean | SD | CV | ARV | ||||
|---|---|---|---|---|---|---|---|---|
| β Value (95%CI) p Value | P interaction | β Value (95%CI) p Value | P interaction | β Value (95%CI) p Value | P interaction | β Value (95%CI) p Value | P interaction | |
| SBP | ||||||||
| Minor stroke b | 7.05 (1.76, 12.33) 0.009 | 0.491 | 2.49 (1.45, 3.53) <0.001 | 0.490 | 1.36 (0.64, 2.07) <0.001 | 0.277 | 2.19 (1.24, 3.14) <0.001 | 0.585 |
| Major stroke b | 9.28 (3.96, 14.61) <0.001 | 2.18 (1.00, 3.35) <0.001 | 0.96 (0.20, 1.71) 0.013 | 1.83 (0.82, 2.85) <0.001 | ||||
| DBP | ||||||||
| Minor stroke b | 0.66 (−2.53, 3.86) 0.682 | 0.554 | 1.07 (0.28, 1.86) 0.008 | 0.580 | 1.41 (0.39, 2.43) 0.007 | 0.302 | 0.93 (0.22, 1.64) 0.010 | 0.999 |
| Major stroke b | 2.76 (−0.96, 6.48) 0.144 | 0.82 (0.03, 1.61) 0.043 | 0.66 (−0.36, 1.67) 0.202 | 0.80 (0.07, 1.52) 0.032 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Fu, Z.; Wu, W.; Zhang, J.; Liu, M.; Gao, L. Association between High-Sensitivity C-Reactive Protein and Blood Pressure Variability in Subacute Stage of Ischemic Stroke. Brain Sci. 2023, 13, 998. https://doi.org/10.3390/brainsci13070998
Xu C, Fu Z, Wu W, Zhang J, Liu M, Gao L. Association between High-Sensitivity C-Reactive Protein and Blood Pressure Variability in Subacute Stage of Ischemic Stroke. Brain Sciences. 2023; 13(7):998. https://doi.org/10.3390/brainsci13070998
Chicago/Turabian StyleXu, Chuanli, Zhiyong Fu, Wei Wu, Jin Zhang, Meitong Liu, and Lianbo Gao. 2023. "Association between High-Sensitivity C-Reactive Protein and Blood Pressure Variability in Subacute Stage of Ischemic Stroke" Brain Sciences 13, no. 7: 998. https://doi.org/10.3390/brainsci13070998