Nanoencapsulated Curcumin: Enhanced Efficacy in Reversing Memory Loss in An Alzheimer Disease Model
Abstract
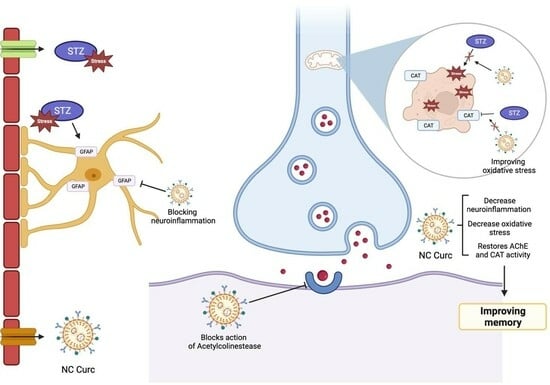
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Formulations
2.3. Surgical Procedure and Experimental Protocol
- (I).
- Control—vehicle (HBSS, 3 μL/ventricule, icv) + unloaded NC (gavage) (n = 12)
- (II).
- Curc—vehicle (HBSS, 3 μL/ventricule, icv) + free curcumin (in canola oil, 6 mg/kg, gavage) (n = 11)
- (III).
- NC Curc—vehicle (HBSS, 3 μL/ventricule, icv) + curcumin-loaded NC (6 mg/kg, gavage) (n = 12)
- (IV).
- STZ—streptozotocin (3 mg/kg, 3 μL/ventricule, icv) + unloaded NC (gavage) (n = 9)
- (V).
- STZ + Curc—streptozotocin (3 mg/kg, 3 μL/ventricule, icv) + free curcumin (in canola oil, 6 mg/kg, gavage) (n = 9)
- (VI).
- STZ + NC Curc—streptozotocin (3 mg/kg, icv) + curcumin-loaded NC (6 mg/kg, gavage) (n = 9)
2.4. Behavioral Tests
2.4.1. Open Field Test
2.4.2. Object Recognition Test
2.4.3. Y-Maze Test
2.4.4. Inhibitory Avoidance Test
2.5. Sample Preparation
2.6. Oxidative Stress Parameters
2.6.1. Reactive Species
2.6.2. Non-Protein Thiol Levels
2.6.3. Thiobarbituric Acid Reactive Species Levels
2.6.4. Catalase Activity
2.7. Acetylcholinesterase Activity
2.8. Immunohistochemistry Assay
2.9. Protein Levels
2.10. Statistical Analysis
3. Results
3.1. Behavioral Tests
3.2. Oxidative Stress Markers
3.3. Acetylcholinesterase Activity
3.4. Neuroinflamation Marker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanojevic, J.B.; Zeljkovic, M.; Dragic, M.; Stojanovic, I.R.; Ilic, T.V.; Stevanovic, I.D.; Ninkovic, M.B. Intermittent theta burst stimulation attenuates oxidative stress and reactive astrogliosis in the streptozotocin-induced model of Alzheimer’s disease-like pathology. Front. Aging Neurosci. 2023, 15, 1161678. [Google Scholar] [CrossRef]
- Latina, V.; Giacovazzo, G.; Calissano, P.; Atlante, A.; La Regina, F.; Malerba, F.; Dell’Aquila, M.; Stigliano, E.; Balzamino, B.O.; Micera, A.; et al. Tau cleavage contributes to cognitive dysfunction in strepto-zotocin-induced sporadic Alzheimer’s disease (sAD) mouse model. Int. J. Mol. Sci. 2021, 22, 12158. [Google Scholar] [CrossRef]
- Sahraei, R.; Aminyavari, S.; Hosseini, M.; Hassanzadeh-Taheri, M.; Foadoddini, M.; Saebipour, M.R. The ameliorative impact of Centella asiatica on the working memory deficit in streptozotocin-induced rat model of Alzheimer disease. Basic Clin. Neurosci. 2022, 13, 25–34. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef]
- Janeczek, M.; Gefen, T.; Samimi, M.; Kim, G.; Weintraub, S.; Bigio, E.; Rogalski, E.; Mesulam, M.M.; Geula, C. Variations in acetylcholinesterase activity within human cortical pyramidal neurons across age and cognitive trajectories. Cereb. Cortex 2018, 28, 1329–1337. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Chen, X.; Xu, Y.; Li, Z.; Yang, Y.; Du, X.; Jiang, Z.; Ni, H. Simultaneous inhibitory effects of all-trans astaxanthin on acetylcholinesterase and oxidative stress. Mar. Drugs 2022, 20, 247. [Google Scholar] [CrossRef]
- Gomes, T.L.N.; Zenha, R.S.S.; Antunes, A.H.; Faria, F.R.; Rezende, K.R.; de Souza, E.L.; Mota, J.F. Evaluation of the impact of different doses of Curcuma longa L. on antioxidant capacity: A randomized, double-blind, crossover pilot trial. BioMed Res. Int. 2021, 2021, 3532864. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Taghibiglou, C. The mechanisms of action of curcumin in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2013, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Li, J.; Chen, C.; Shan, Z.; Cao, X.; Zhou, X.F.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part II: In vivo safety and efficacy assessment against behavior deficit in Alzheimer disease. Drug Deliv. Transl. Res. 2018, 8, 1406–1420. [Google Scholar] [CrossRef]
- Fidelis, E.M.; Savall, A.S.P.; da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Bazanella Sampaio, T.; Pinton, S. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by β-amyloid in mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.Z.; Savall, A.S.P.; da Luz Abreu, E.; Nakama, K.A.; Dos Santos, R.B.; Guedes, M.C.M.; Ávila, D.S.; Luchese, C.; Haas, S.E.; Quines, C.B.; et al. Co-nanoencapsulated meloxicam and curcumin improves cognitive impairment induced by amyloid-beta through modulation of cyclooxygenase-2 in mice. Neural Regen. Res. 2021, 16, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.A.; Hosny, E.N.; Khadrawy, Y.A.; Mourad, I.M.; Othman, A.I.; Aboul Ezz, H.S.; Mohammed, H.S. Effect of curcumin nanoparticles on streptozotocin-induced male Wistar rat model of Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Rucińska, E.; Ruciński, J.; Myślińska, D.; Grembecka, B.; Wrona, D.; Majkutewicz, I. Dimethyl fumarate alleviates adult neurogenesis disruption in hippocampus and olfactory bulb and spatial cognitive deficits induced by intracerebroventricular streptozotocin injection in young and aged rats. Int. J. Mol. Sci. 2022, 23, 15449. [Google Scholar] [CrossRef] [PubMed]
- Pinton, S.; Sampaio, T.B.; Ramalho, R.M.; Rodrigues, C.M.; Nogueira, C.W. p,p′-Methoxyl-diphenyl diselenide prevents neurodegeneration and glial cell activation induced by streptozotocin in rats. J. Alzheimer’s Dis. JAD 2013, 33, 133–144. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Tucker, D.; Dong, Y.; Zhu, L.; Duan, R.; Liu, T.C.; Zhang, Q. Beneficial effects of exercise pretreatment in a sporadic Alzheimer’s rat model. Med. Sci. Sports Exerc. 2018, 50, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Biasibetti, R.; Almeida Dos Santos, J.P.; Rodrigues, L.; Wartchow, K.M.; Suardi, L.Z.; Nardin, P.; Selistre, N.G.; Vázquez, D.; Gonçalves, C.A. Hippocampal changes in STZ-model of Alzheimer’s disease are dependent on sex. Behav. Brain Res. 2017, 316, 205–214. [Google Scholar] [CrossRef]
- Santos, R.B.D.; Nakama, K.A.; Pacheco, C.O.; de Gomes, M.G.; de Souza, J.F.; de Souza Pinto, A.C.; de Oliveira, F.A.; da Fonseca, A.L.; Varotti, F.; Fajardo, A.R.; et al. Curcumin-loaded nanocapsules: Influence of surface characteristics on technological parameters and potential antimalarial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111356. [Google Scholar] [CrossRef]
- Loch-Neckel, G.; Santos-Bubniak, L.; Mazzarino, L.; Jacques, A.V.; Moccelin, B.; Santos-Silva, M.C.; Lemos-Senna, E. Orally administered chitosan-coated polycaprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J. Pharm. Sci. 2015, 104, 3524–3534. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D.; Galea, L.A.; Kuroda, Y.; McEwen, B.S. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996, 110, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D.; Lupien, S.J.; Thanasoulis, L.C.; McEwen, B.S. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res. 1997, 759, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Loetchutinat, C.; Kothan, S.; Dechsupa, S.; Meesungnoe, J.; Jay-Gerin, J.P.; Mankhetkorn, S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat. Phys. Chem. 2005, 72, 323–331. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pinz, M.P.; de Oliveira, R.L.; da Fonseca, C.A.R.; Voss, G.T.; da Silva, B.P.; Duarte, L.F.B.; Domingues, W.B.; Ortiz, H.G.; Savall, A.S.P.; Meotti, F.C.; et al. A purine derivative containing an organoselenium group protects against memory impairment, sensitivity to nociception, oxidative damage, and neuroinflammation in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2023, 60, 1214–1231. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Grünblatt, E.; Salkovic-Petrisic, M.; Osmanovic, J.; Riederer, P.; Hoyer, S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J. Neurochem. 2007, 101, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Nabavi Zadeh, F.; Nazari, M.; Amini, A.; Adeli, S.; Barzegar Behrooz, A.; Fahanik Babaei, J. Pre- and post-treatment of α-Tocopherol on cognitive, synaptic plasticity, and mitochondrial disorders of the hippocampus in icv-streptozotocin-induced sporadic Alzheimer’s-like disease in male Wistar rat. Front. Neurosci. 2023, 17, 1073369. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Bona, N.P.; Soares, M.S.P.; Teixeira, F.C.; Rahmeier, F.L.; Carvalho, F.B.; da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales, R.A.; et al. Tannic acid ameliorates STZ-induced Alzheimer’s disease-like impairment of memory, neuroinflammation, neuronal death and modulates Akt expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef]
- Gáspár, A.; Hutka, B.; Ernyey, A.J.; Tajti, B.T.; Varga, B.T.; Zádori, Z.S.; Gyertyán, I. Performance of the intracerebroventricularly injected streptozotocin Alzheimer’s disease model in a translationally relevant, aged and experienced rat population. Sci. Rep. 2022, 12, 20247. [Google Scholar] [CrossRef]
- Pinz, M.P.; Vogt, A.G.; da Costa Rodrigues, K.; Dos Reis, A.S.; Duarte, L.F.B.; Fronza, M.G.; Domingues, W.B.; Blodorn, E.B.; Alves, D.; Campos, V.F.; et al. Effect of a purine derivative containing selenium to improve memory decline and anxiety through modulation of the cholinergic system and Na+/K+-ATPase in an Alzheimer’s disease model. Metab. Brain Dis. 2021, 36, 871–888. [Google Scholar] [CrossRef]
- Lannert, H.; Hoyer, S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci. 1998, 112, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lu, S.; Liu, X.G.; Zhu, J.; Wang, Y.J.; Liu, R.T. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.B.; de Gomes, M.G.; Savall, A.S.P.; Fidelis, E.M.; Pinton, S.; Ribeiro, A.C.F.; Munieweg, F.R.; Oelke, C.A.; Haas, S.E. Evaluation of curcumin-loaded polymeric nanocapsules with different coatings in chick embryo model: Influence on angiogenesis, teratogenesis and oxidative stress. Pharmacol. Rep. PR 2021, 73, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, A. Comparative analysis of intrahippocampal amyloid beta (1-42) and it is intracerebroventricular streptozotocin models of Alzheimer’s disease: Possible behavioral, biochemical, mitochondrial, cellular and histopathological evidences. J. Alzheimers Dis. Park. 2016, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Gutierres, J.M.; Carvalho, F.; Lopes, T.F.; Antunes, V.; da Costa, P.; Pereira, M.E.; Schetinger, M.R.C.; Morsch, V.M.; de Andrade, C.M. Protection of cholinergic and antioxidant system contributes to the effect of vitamin D3 ameliorating memory dysfunction in sporadic dementia of Alzheimer’s type. Redox Rep. Commun. Free Radic. Res. 2019, 24, 34–40. [Google Scholar] [CrossRef]
- Rodrigues, K.D.C.; Neto, M.R.D.S.; Barboza, V.D.S.; Hass, S.E.; Vaucher, R.D.A.; Giongo, J.L.; Schumacher, R.F.; Wilhelm, R.A.; Luchese, C. Anti-amnesic, antidepressant, and anxiolytic-like responses of curcumin-loaded nanocapsules in mice: Modulating acetylcholinesterase activity, oxidative parameters, and neuroinflammation biomarkers. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Venkateshappa, C.; Harish, G.; Mahadevan, A.; Srinivas Bharath, M.M.; Shankar, S.K. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: Implications for neurodegeneration in Alzheimer’s disease. Neurochem. Res. 2012, 37, 1601–1614. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 9613090. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target enzyme in Alzheimer’s disease: Acetylcholinesterase inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Abbasi, M.A.; Ilyas, M.; Rehman-ur-Aziz; Sonia, A.; Shahwar, D.; Raza, M.A.; Khan, K.M.; Ashraf, M.; Afzal, I.; Ambreen, N. Curcumin and its derivatives: Moderate inhibitors of acetylcholinesterase, butyrylcholinesterase and trypsin. Sci. Iran. 2012, 19, 1580–1583. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Okonkwo, P.K.; Faboya, O.A.; Onikanni, S.A.; Fadaka, A.; Olayide, I.; Akinyemi, E.O.; Oboh, G. Curcumin improves episodic memory in cadmium induced memory impairment through inhibition of acetylcholinesterase and adenosine deaminase activities in a rat model. Metab. Brain Dis. 2017, 32, 87–95. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lee, C.J.; Chen, L.C.; Lee, T.L.; Hsieh, Y.Y.; Han, C.H.; Yang, C.H.; Huang, W.J.; Hou, W.C. Acetylcholinesterase inhibitory activity and neuroprotection in vitro, molecular docking, and improved learning and memory functions of demethylcurcumin in scopolamine-induced amnesia ICR mice. Food Funct. 2020, 11, 2328–2338. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Z.; Tian, Z.; Blanchard, J.; Dai, C.L.; Chalbot, S.; Iqbal, K.; Liu, F.; Gong, C.X. Intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol. Neurobiol. 2014, 49, 547–562. [Google Scholar] [CrossRef]
- Knezovic, A.; Loncar, A.; Homolak, J.; Smailovic, U.; Osmanovic Barilar, J.; Ganoci, L.; Bozina, N.; Riederer, P.; Salkovic-Petrisic, M. Rat brain glucose transporter-2, insulin receptor and glial expression are acute targets of intracerebroventricular streptozotocin: Risk factors for sporadic Alzheimer’s disease? J. Neural Transm. 2017, 124, 695–708. [Google Scholar] [CrossRef]
- Hoppe, J.B.; Coradini, K.; Frozza, R.L.; Oliveira, C.M.; Meneghetti, A.B.; Bernardi, A.; Pires, E.S.; Beck, R.C.; Salbego, C.G. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: Involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol. Learn. Mem. 2013, 106, 134–144. [Google Scholar] [CrossRef]






| Open Field Test | Y-Maze Test | |||
|---|---|---|---|---|
| Crossing | Rearing | Arm Entries | Alternations | |
| Control | 48.38 ± 24.38 | 25.54 ± 18.18 | 14.45 ± 5.02 | 53.73 ± 20.30 |
| Curc | 46.50 ± 23.03 | 21.55 ± 12.50 | 13.39 ± 3.96 | 45.18 ± 14.86 |
| NC Curc | 46.00 ± 28.64 | 23.58 ± 18.00 | 13.25 ± 4.71 | 50.67 ± 19.66 |
| STZ | 48.72 ± 23.19 | 27.28 ± 16.38 | 16.89 ± 5.77 | 52.46 ± 12.76 |
| STZ + Curc | 57.78 ± 28.64 | 20.33 ± 9.77 | 16.13 ± 5.90 | 37.63 ± 8.18 |
| STZ + NC Curc | 34.19 ± 25.52 | 17.06 ± 14.79 | 12.75 ± 3.73 | 52.23 ± 14.74 |
| RS | TBARS | |||
|---|---|---|---|---|
| Prefrontal Cortex | Hippocampus | Prefrontal Cortex | Hippocampus | |
| Control | 287.4 ± 193 | 498.5 ± 304 | 1.257 ± 0.28 | 1.211 ± 0.31 |
| Curc | 272.6 ± 235 | 546.5 ± 206 | 1.345 ± 0.52 | 1.125 ± 0.35 |
| NC Curc | 368.6 ± 335 | 423.8 ± 265 | 1.175 ± 0.39 | 1.128 ± 0.29 |
| STZ | 267.5 ± 144 | 516.1 ± 276 | 1.123 ± 0.33 | 0.954 ± 0.31 |
| STZ + Curc | 270.4 ± 158 | 485.9 ± 269 | 1.205 ± 0.65 | 1.359 ± 0.37 |
| STZ + NC Curc | 298.6 ± 180 | 464.2 ± 382 | 1.370 ± 0.42 | 1.076 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savall, A.S.P.; de Mello, J.D.; Fidelis, E.M.; Comis-Neto, A.A.; Nepomuceno, M.R.; Pacheco, C.d.O.; Haas, S.E.; Pinton, S. Nanoencapsulated Curcumin: Enhanced Efficacy in Reversing Memory Loss in An Alzheimer Disease Model. Brain Sci. 2024, 14, 130. https://doi.org/10.3390/brainsci14020130
Savall ASP, de Mello JD, Fidelis EM, Comis-Neto AA, Nepomuceno MR, Pacheco CdO, Haas SE, Pinton S. Nanoencapsulated Curcumin: Enhanced Efficacy in Reversing Memory Loss in An Alzheimer Disease Model. Brain Sciences. 2024; 14(2):130. https://doi.org/10.3390/brainsci14020130
Chicago/Turabian StyleSavall, Anne Suély Pinto, Jhuly Dorneles de Mello, Eduarda Monteiro Fidelis, Antonio Alvenir Comis-Neto, Maria Regina Nepomuceno, Camila de Oliveira Pacheco, Sandra Elisa Haas, and Simone Pinton. 2024. "Nanoencapsulated Curcumin: Enhanced Efficacy in Reversing Memory Loss in An Alzheimer Disease Model" Brain Sciences 14, no. 2: 130. https://doi.org/10.3390/brainsci14020130