Exoscopic Microsurgery: A Change of Paradigm in Brain Tumor Surgery? Comparison with Standard Operative Microscope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Radiological Data
2.5. Neuro-Oncological Treatment and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Overall Outcome
3.3. Exoscope and Microscope Comparison
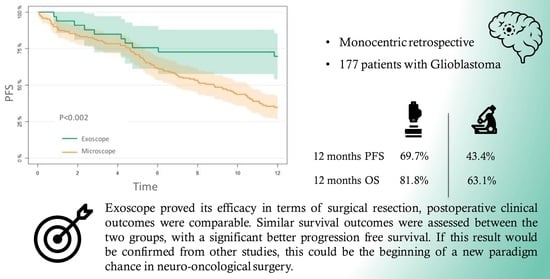
3.4. PFS
3.5. OS
3.6. Cox Model
4. Discussion
4.1. Surgical Resection of Gliomas: Implication of Introduction of a 3D Exoscope
4.2. Our Experience
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardo, A. The Changing Face of Technologically Integrated Neurosurgery: Today’s High-Tech Operating Room. World Neurosurg. 2017, 106, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Javed, G.; Mubeen, B.; Bareeqa, S.B.; Rasheed, H.; Rehman, A.; Phulpoto, M.M.; Samar, S.S.; Aziz, K. Robotics in neurosurgery: A literature review. J. Pak. Med. Assoc. 2018, 68, 258–263. [Google Scholar]
- Senders, J.T.; Arnaout, O.; Karhade, A.V.; Dasenbrock, H.H.; Gormley, W.B.; Broekman, M.L.; Smith, T.R. Natural and Artificial Intelligence in Neurosurgery: A Systematic Review. Neurosurgery 2018, 83, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uluç, K.; Kujoth, G.C.; Başkaya, M.K. Operating microscopes: Past, present, and future. Neurosurg. Focus 2009, 27, E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA. Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro. Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Youngblood, M.W.; Stupp, R.; Sonabend, A.M. Role of Resection in Glioblastoma Management. Neurosurg. Clin. N. Am. 2021, 32, 9–22. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Yordanova, Y.N.; Duffau, H. Supratotal resection of diffuse gliomas—An overview of its multifaceted implications. Neurochirurgie 2017, 63, 243–249. [Google Scholar] [CrossRef]
- Duffau, H. Is supratotal resection of glioblastoma in noneloquent areas possible? World Neurosurg. 2014, 82, e101–e103. [Google Scholar] [CrossRef] [PubMed]
- Certo, F.; Stummer, W.; Farah, J.O.; Freyschlag, C.; Visocchi, M.; Morrone, A.; Altieri, R.; Toccaceli, G.; Peschillo, S.; Thomè, C.; et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J. Neurosurg. Sci. 2019, 63, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Giussani, C.; Carrabba, G.; Rui, C.B.; Chiarello, G.; Stefanoni, G.; Julita, C.; De Vito, A.; Cinalli, M.A.; Basso, G.; Remida, P.; et al. Perilesional resection technique of glioblastoma: Intraoperative ultrasound and histological findings of the resection borders in a single center experience. J. Neurooncol. 2023, 161, 625–632. [Google Scholar] [CrossRef]
- Di Cristofori, A.; Basso, G.; de Laurentis, C.; Mauri, I.; Sirtori, M.A.; Ferrarese, C.; Isella, V.; Giussani, C. Perspectives on (A)symmetry of Arcuate Fasciculus. A Short Review About Anatomy, Tractography and TMS for Arcuate Fasciculus Reconstruction in Planning Surgery for Gliomas in Language Areas. Front. Neurol. 2021, 12, 639822. [Google Scholar] [CrossRef]
- Ricciardi, L.; Chaichana, K.L.; Cardia, A.; Stifano, V.; Rossini, Z.; Olivi, A.; Sturiale, C.L. The Exoscope in Neurosurgery: An Innovative “Point of View”. A Systematic Review of the Technical, Surgical, and Educational Aspects. World Neurosurg. 2019, 124, 136–144. [Google Scholar] [CrossRef]
- Fiani, B.; Jarrah, R.; Griepp, D.W.; Adukuzhiyil, J. The Role of 3D Exoscope Systems in Neurosurgery: An Optical Innovation. Cureus 2021, 13, e15878. [Google Scholar] [CrossRef]
- Baron, R.B.; Lakomkin, N.; Schupper, A.J.; Nistal, D.; Nael, K.; Price, G.; Hadjipanayis, C.G. Postoperative outcomes following glioblastoma resection using a robot-assisted digital surgical exoscope: A case series. J. Neurooncol. 2020, 148, 519–527. [Google Scholar] [CrossRef]
- Calloni, T.; Antolini, L.; Roumy, L.-G.; Nicolosi, F.; Carrabba, G.G.; Di Cristofori, A.; Fontanella, M.M.; Giussani, C.G. Exoscope and operative microscope for training in microneurosurgery: A laboratory investigation on a model of cranial approach. Front. Surg. 2023, 10, 1150981. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W.J.; Grady, M.S.; Gabel, B.C.; Cohen, M.A.; Heuer, G.G.; Pisapia, J.; Bohman, L.-E.; Leibowitz, J.M. Comparison of endoscopic and microscopic removal of pituitary adenomas: Single-surgeon experience and the learning curve. Neurosurg. Focus 2008, 25, E10. [Google Scholar] [CrossRef] [Green Version]
- Lorentzen, H.; Weismann, K.; Secher, L.; Petersen, C.S.; Larsen, F.G. The dermatoscopic ABCD rule does not improve diagnostic accuracy of malignant melanoma. Acta Derm. Venereol. 1999, 79, 469–472. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Scerrati, A.; Ricciardi, L.; Trevisi, G. The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. J. Clin. Med. 2021, 11, 223. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Mattogno, P.; Menna, G.; Agostini, L.; Olivi, A.; Doglietto, F. A Comparative Analysis with Exoscope and Optical Microscope for Intraoperative Visualization and Surgical Workflow in 5-Aminolevulinic Acid-Guided Resection of High-Grade Gliomas. World Neurosurg. 2023, 170, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Abunimer, A.M.; Abou-Al-Shaar, H.; White, T.G.; Park, J.; Schulder, M. The Utility of High-Definition 2-Dimensional Stereotactic Exoscope in Cranial and Spinal Procedures. World Neurosurg. 2022, 158, e231–e236. [Google Scholar] [CrossRef]
- Keric, N.; Krenzlin, H.; Kurz, E.; Wesp, D.M.A.; Kalasauskas, D.; Ringel, F. Evaluation of 3D Robotic-Guided Exoscopic Visualization in Microneurosurgery. Front. Surg. 2021, 8, 791427. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Kroll, D.; Etame, A.; Tran, N.; Liu, J.; Ford, A.; Sparr, E.; Kim, Y.; Forsyth, P.; Sahebjam, S.; et al. A Prospective Validation Study of the First 3D Digital Exoscope for Visualization of 5-ALA-Induced Fluorescence in High-Grade Gliomas. World Neurosurg. 2021, 149, e498–e503. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Drazin, D.; Shirzadi, A.; Black, K.L.; Berci, G. Infratentorial supracerebellar resection of a pineal tumor using a high definition video exoscope (VITOM®). J. Clin. Neurosci. 2012, 19, 306–309. [Google Scholar] [CrossRef]
- Birch, K.; Drazin, D.; Black, K.L.; Williams, J.; Berci, G.; Mamelak, A.N. Clinical experience with a high definition exoscope system for surgery of pineal region lesions. J. Clin. Neurosci. 2014, 21, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.X.; Witten, A.J.; Shah, M. V Resection of a pineal region papillary tumor using robotic exoscope: Improved visualization and ergonomics for deep seeded tumor. Neurosurg. Focus Video 2021, 5, V14. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Eskandari, R.; Kosnik-Infinger, L.; Olivera, R.; Nangunoori, R.; Patel, S.; Williamson, R.; Yu, A.; Hadjipanayis, C.G. A Multicenter Study Investigating the Surgeon Experience with a Robotic-Assisted Exoscope as Part of the Neurosurgical Armamentarium. World Neurosurg. 2023, 173, e571–e577. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Iwami, K.; Kishida, Y.; Nagatani, T.; Yatsuya, H.; Miyachi, S. Combined Exoscopic and Endoscopic Two-Step Keyhole Approach for Intracranial Meningiomas. Curr. Oncol. 2022, 29, 5370–5382. [Google Scholar] [CrossRef]
- Haeren, R.; Hafez, A.; Lehecka, M. Visualization and Maneuverability Features of a Robotic Arm Three-Dimensional Exoscope and Operating Microscope for Clipping an Unruptured Intracranial Aneurysm: Video Comparison and Technical Evaluation. Oper. Neurosurg. 2022, 22, 28–34. [Google Scholar] [CrossRef]
- Calloni, T.; Roumy, L.G.; Cinalli, M.A.; Rocca, A.; Held, A.; Trezza, A.; Carrabba, G.G.; Giussani, C.G. Exoscope as a Teaching Tool: A Narrative Review of the Literature. Front. Surg. 2022, 9, 878293. [Google Scholar] [CrossRef]
- Chu, T.-S.; Chu, T.-H.; Huynh, T.-D.; Phan, V.-D.; Dang, B.N.; Tran, Q.D. Radical resection of trigeminal schwannoma at the cerebellopontine angle with support of the digital robotic exoscope Synaptive Modus V system: A case report and literature review. Medicine 2023, 102, e33492. [Google Scholar] [CrossRef]
- Schupper, A.J.; Roa, J.A.; Hadjipanayis, C.G. Contemporary intraoperative visualization for GBM with use of exoscope, 5-ALA fluorescence-guided surgery and tractography. Neurosurg. Focus Video 2022, 6, V5. [Google Scholar] [CrossRef]
- Maeda, M.; Nonaka, M.; Naito, N.; Ueno, K.; Kamei, T.; Asai, A. 5-ALA fluorescence-guided resection of pediatric low-grade glioma using the ORBEYE 3D digital exoscope: A technical report. Child’s Nerv. Syst. 2023, 39, 1061–1064. [Google Scholar] [CrossRef]
- Beez, T.; Munoz-Bendix, C.; Beseoglu, K.; Steiger, H.-J.; Ahmadi, S.A. First Clinical Applications of a High-Definition Three-Dimensional Exoscope in Pediatric Neurosurgery. Cureus 2018, 10, e2108. [Google Scholar] [CrossRef] [Green Version]
- Gassie, K.; Wijesekera, O.; Chaichana, K.L. Minimally invasive tubular retractor-assisted biopsy and resection of subcortical intra-axial gliomas and other neoplasms. J. Neurosurg. Sci. 2018, 62, 682–689. [Google Scholar] [CrossRef]
- Ikeda, N.; Furuse, M.; Futamura, G.; Kimura, S.; Nonoguchi, N.; Kawabata, S.; Kameda, M.; Yokoyama, K.; Takami, T.; Kawanishi, M.; et al. The Characteristic of Light Sources and Fluorescence in the 3-Dimensional Digital Exoscope “ORBEYE” for 5-Aminolevulinic Acid-Induced Fluorescence-Guided Surgery Compared with a Conventional Microscope. World Neurosurg. 2022, 167, e1268–e1274. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Toda, M.; Nishimoto, M.; Ishihara, E.; Miwa, T.; Akiyama, T.; Horiguchi, T.; Sasaki, H.; Yoshida, K. Pros and cons of using ORBEYETM for microneurosurgery. Clin. Neurol. Neurosurg. 2018, 174, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Khalessi, A.A.; Rahme, R.; Rennert, R.C.; Borgas, P.; Steinberg, J.A.; White, T.G.; Santiago-Dieppa, D.R.; Boockvar, J.A.; Hatefi, D.; Pannell, J.S.; et al. First-in-Man Clinical Experience Using a High-Definition 3-Dimensional Exoscope System for Microneurosurgery. Oper. Neurosurg. 2019, 16, 717–725. [Google Scholar] [CrossRef]
- Muscas, G.; Battista, F.; Boschi, A.; Morone, F.; Della Puppa, A. A Single-Center Experience with the Olympus ORBEYE 4K-3D Exoscope for Microsurgery of Complex Cranial Cases: Technical Nuances and Learning Curve. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 484–489. [Google Scholar] [CrossRef]
- Haglund, M.M.; Berger, M.S.; Shamseldin, M.; Lettich, E.; Ojemann, G.A. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery 1994, 34, 567–576; discussion 576. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Del Bene, M.; Moiraghi, A.; Casali, C.; Legnani, F.G.; Saladino, A.; Perin, A.; Vetrano, I.G.; Mattei, L.; Richetta, C.; et al. From Grey Scale B-Mode to Elastosonography: Multimodal Ultrasound Imaging in Meningioma Surgery-Pictorial Essay and Literature Review. Biomed Res. Int. 2015, 2015, 925729. [Google Scholar] [CrossRef]
- Strickland, B.A.; Zada, G. 5-ALA Enhanced Fluorescence-Guided Microscopic to Endoscopic Resection of Deep Frontal Subcortical Glioblastoma Multiforme. World Neurosurg. 2021, 148, 65. [Google Scholar] [CrossRef]
- Duffau, H. Intraoperative direct subcortical stimulation for identification of the internal capsule, combined with an image-guided stereotactic system during surgery for basal ganglia lesions. Surg. Neurol. 2000, 53, 250–254. [Google Scholar] [CrossRef]
- Bello, L.; Gallucci, M.; Fava, M.; Carrabba, G.; Giussani, C.; Acerbi, F.; Baratta, P.; Songa, V.; Conte, V.; Branca, V.; et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 2007, 60, 62–67. [Google Scholar] [CrossRef]
- Bello, L.; Acerbi, F.; Giussani, C.; Baratta, P.; Taccone, P.; Songa, V.; Fava, M.; Stocchetti, N.; Papagno, C.; Gaini, S.M. Intraoperative language localization in multilingual patients with gliomas. Neurosurgery 2006, 59, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Orringer, D.A.; Golby, A.; Jolesz, F. Neuronavigation in the surgical management of brain tumors: Current and future trends. Expert Rev. Med. Devices 2012, 9, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Rohde, V.; Spangenberg, P.; Mayfrank, L.; Reinges, M.; Gilsbach, J.M.; Coenen, V.A. Advanced neuronavigation in skull base tumors and vascular lesions. Minim. Invasive Neurosurg. 2005, 48, 13–18. [Google Scholar] [CrossRef]
- Veiceschi, P.; Locatelli, D.; Dario, A.; Agresta, G. Frameless neuronavigation-assisted brain biopsy with electromagnetic tracking: How I do it? Acta Neurochir. 2022, 164, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Saito, R. Intraoperative Functional Monitoring in Brain Tumor Surgery. No Shinkei Geka. 2023, 51, 481–489. [Google Scholar] [CrossRef]
- Shao, K.N.; Chen, S.S.; Lee, L.S. Intraoperative neurosurgical ultrasonography. Zhonghua Yi Xue Za Zhi 1999, 62, 775–781. [Google Scholar]
- Prada, F.; Del Bene, M.; Mattei, L.; Lodigiani, L.; DeBeni, S.; Kolev, V.; Vetrano, I.; Solbiati, L.; Sakas, G.; DiMeco, F. Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med. 2015, 36, 174–186. [Google Scholar] [CrossRef]
- Regelsberger, J.; Fritzsche, E.; Langer, N.; Westphal, M. Intraoperative sonography of intra- and extramedullary tumors. Ultrasound Med. Biol. 2005, 31, 593–598. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Rampini, A.; Mattei, L.; Casali, C.; Vetrano, I.G.; Gennari, A.G.; Sdao, S.; Saini, M.; Sconfienza, L.M.; et al. Intraoperative Strain Elastosonography in Brain Tumor Surgery. Oper. Neurosurg. 2019, 17, 227–236. [Google Scholar] [CrossRef]
- Seifert, V. Intraoperative MRI in neurosurgery: Technical overkill or the future of brain surgery? Neurol. India 2003, 51, 329–332. [Google Scholar]
- Nabavi, A.; Dörner, L.; Stark, A.M.; Mehdorn, H.M. Intraoperative MRI with 1.5 Tesla in neurosurgery. Neurosurg. Clin. N. Am. 2009, 20, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofori, A.; Carone, G.; Rocca, A.; Rui, C.B.; Trezza, A.; Carrabba, G.; Giussani, C. Fluorescence and Intraoperative Ultrasound as Surgical Adjuncts for Brain Metastases Resection: What Do We Know? A Systematic Review of the Literature. Cancers 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, P.; Zhang, M.; Miller, K.J.; Robe, P.; Li, G. How Intraoperative Tools and Techniques Have Changed the Approach to Brain Tumor Surgery. Curr. Oncol. Rep. 2018, 20, 89. [Google Scholar] [CrossRef]
- Witten, A.J.; Ben-Shalom, N.; Ellis, J.A.; Boockvar, J.A.; D’Amico, R.S. Optimization of novel exoscopic blue light filter during fluorescence-guided resection of Glioblastoma. J. Neurooncol. 2023, 161, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Murai, Y.; Sato, S.; Yui, K.; Morimoto, D.; Ozeki, T.; Yamaguchi, M.; Tateyama, K.; Nozaki, T.; Tahara, S.; Yamaguchi, F.; et al. Preliminary Clinical Microneurosurgical Experience With the 4K3-Dimensional Microvideoscope (ORBEYE) System for Microneurological Surgery: Observation Study. Oper. Neurosurg. 2019, 16, 707–716. [Google Scholar] [CrossRef]
- Doglietto, F.; Belotti, F.; Panciani, P.; Poliani, P.L.; Fontanella, M.M. High-Definition 3-Dimensional Exoscope for 5-ALA Glioma Surgery: 3-Dimensional Operative Video. Oper. Neurosurg. 2020, 18, E82. [Google Scholar] [CrossRef]
- Belloch, J.P.; Rovira, V.; Llácer, J.L.; Riesgo, P.A.; Cremades, A. Fluorescence-guided surgery in high grade gliomas using an exoscope system. Acta Neurochir. 2014, 156, 653–660. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Suarez-Meade, P.; Chaichana, K.L. Bur Hole-Based Resections of Intrinsic Brain Tumors with Exoscopic Visualization. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 105–111. [Google Scholar] [CrossRef]
- Piquer, J.; Llácer, J.L.; Rovira, V.; Riesgo, P.; Rodriguez, R.; Cremades, A. Fluorescence-guided surgery and biopsy in gliomas with an exoscope system. Biomed Res. Int. 2014, 2014, 207974. [Google Scholar] [CrossRef] [Green Version]
- Roethe, A.L.; Landgraf, P.; Schröder, T.; Misch, M.; Vajkoczy, P.; Picht, T. Monitor-based exoscopic 3D4k neurosurgical interventions: A two-phase prospective-randomized clinical evaluation of a novel hybrid device. Acta Neurochir. 2020, 162, 2949–2961. [Google Scholar] [CrossRef]




| Overall | EXOSCOPE | MICROSCOPE | p | |
|---|---|---|---|---|
| n (%) | 177 | 33 (18.6%) | 144 (81.4%) | |
| AGE (median I–III) | 65 (57,73) | 62 (51, 73) | 65 (58, 73) | 0.139 |
| SEX = M, n (%) | 111 (62.7) | 23 (69.7) | 88 (61.1) | 0.471 |
| Experience = LOW, n (%) | 47 (26.6) | 11 (33.3) | 36 (25.0) | 0.448 |
| LOBE, n (%) | 0.749 | |||
| Frontal | 53 (29.9) | 11 (33.3) | 42 (29.2) | |
| Temporal | 53 (29.9) | 9 (27.3) | 44 (30.6) | |
| Insular | 11 (6.2) | 4 (12.1) | 7 (4.9) | |
| Occipital | 24 (13.6) | 4 (12.1) | 20 (13.9) | |
| Parietal | 24 (13.6) | 4 (12.1) | 20 (13.9) | |
| Corpus callosum | 5 (2.8) | 0 (0.0) | 5 (3.5) | |
| Thalamus/pineal | 5 (2.8) | 1 (3.0) | 4 (2.8) | |
| Multifocal | 2 (1.1) | 0 (0.0) | 2 (1.4) | |
| SIDE, n (%) | 0.846 | |||
| Right | 92 (52.0) | 17 (51.5) | 75 (52.1) | |
| Midline | 3 (1.7) | 0 (0.0) | 3 (2.1) | |
| Multifocal | 4 (2.3) | 1 (3.0) | 3 (2.1) | |
| Left | 78 (44.1) | 15 (45.5) | 63 (43.8) | |
| IDH = Mutation, n (%) | 9 (5.2) | 4 (12.1) | 5 (3.6) | 0.120 |
| MGMT = Methylation, n (%) | 76 (44.4) | 10 (30.3) | 66 (47.8) | 0.104 |
| GTR, n (%) | 0.148 | |||
| GTR | 122 (68.9) | 23 (69.7) | 99 (68.8) | |
| PR | 41 (23.2) | 5 (15.2) | 36 (25.0) | |
| STR | 14 (7.9) | 5 (15.2) | 9 (6.2) | |
| KPS ≥ 70, n (%) | 116 (67.8) | 26 (83.9) | 90 (64.3) | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cristofori, A.; Graziano, F.; Rui, C.B.; Rebora, P.; Di Caro, D.; Chiarello, G.; Stefanoni, G.; Julita, C.; Florio, S.; Ferlito, D.; et al. Exoscopic Microsurgery: A Change of Paradigm in Brain Tumor Surgery? Comparison with Standard Operative Microscope. Brain Sci. 2023, 13, 1035. https://doi.org/10.3390/brainsci13071035
Di Cristofori A, Graziano F, Rui CB, Rebora P, Di Caro D, Chiarello G, Stefanoni G, Julita C, Florio S, Ferlito D, et al. Exoscopic Microsurgery: A Change of Paradigm in Brain Tumor Surgery? Comparison with Standard Operative Microscope. Brain Sciences. 2023; 13(7):1035. https://doi.org/10.3390/brainsci13071035
Chicago/Turabian StyleDi Cristofori, Andrea, Francesca Graziano, Chiara Benedetta Rui, Paola Rebora, Diego Di Caro, Gaia Chiarello, Giovanni Stefanoni, Chiara Julita, Santa Florio, Davide Ferlito, and et al. 2023. "Exoscopic Microsurgery: A Change of Paradigm in Brain Tumor Surgery? Comparison with Standard Operative Microscope" Brain Sciences 13, no. 7: 1035. https://doi.org/10.3390/brainsci13071035