1. Introduction
With the rapid development of urbanization, the increase of impervious areas has interrupted the water infiltration channel, which has greatly increased the rainfall runoff and peak discharge [
1]. The heavy metals in urban stormwater runoff mainly come from automobile exhaust, industrial smoke and fossil fuel combustion, dust and various metal facilities. Heavy metals such as copper, zinc and lead are common components in stormwater runoff and usually with relatively high concentrations. Unlike organic pollutants, heavy metals are difficult to degrade in the environment and are easily accumulated in the human body through the food chain. Excessive intake of heavy metals in the human body may irritate the mucous membranes, leading to liver and kidney damage, capillary damage and central nervous system problems [
2]. Cu(II) is essential for some biosyntheses in the human body, and micronutrients as animals and plants are well known, but it is toxic with high concentrations. When the concentration is above the threshold level, it can cause anemia and gastrointestinal discomfort. Infiltration facilities such as permeable pavements could remove pollutants in urban runoff to some extent. Typical pavement systems are mainly composed of materials such as sand and gravel. Because of the unique properties of these media, the adsorption capacity is very limited. The removal of heavy metals in runoff is mainly through the interception of pores in the media. However, with the operation of the permeable paving system, the problem of pore blockage, which is the bottleneck of the operation of the currently trapped system, also results in a significant reduction in the continuous retention of contaminants. In addition, with the long-term operation of the system, the extremely limited adsorption capacity was not able to achieve good removal efficiency for pollutants. Therefore, improving the adsorption capacity of the pavement system medium towards pollutants has a positive effect on reducing the water quality risks.
In many countries, construction and demolition wastes are the main types of wastes in terms of weight. In Europe, more than 30% (
w/
w) of wastes generated is waste bricks. The production of brick-concrete construction wastes in China has been currently increasing year by year. The amount of this type of wastes accounts for 30% to 40% of the total amount of municipal waste. A large amount of construction wastes has piled up, which seriously pollutes the city’s atmosphere, water and soil environment, and has a local impact on people’s production and life. Recently, there has been an increasing interest in using construction wastes as adsorbents to remove pollutants from wastewater or stormwater. Construction waste bricks are used as the packaging media in percolation facilities. Using construction wastes in remediation were proposed as an alternative method to replace the current methods with a relatively high cost [
3]. Due to many economic advantages, construction waste bricks are cheaper than other alternative adsorbents, such as activated carbon, natural and synthetic zeolites, and ion exchange resins. Wilson et al. showed that concrete blocks, crushed stone beds, and geotextiles in the permeable paving system can retain hydrocarbon contaminants and improve the quality of effluent [
4]. Wang et al. confirmed that using construction wastes as bioretention media can remove 90% of heavy metals in rainwater [
1]. Hussain et al. showed that brick kiln chimney wastes could efficiently remove chromium from solutions and the removal capacity could reach 38.8 mg/g [
5].
Although the results of using construction waste bricks to remove heavy metals are promising, there is still a need to better understand the removal efficiency and operational process such as how inlet concentration and residence time affect the removing of pollutants. Nnadi et al. confirmed that a permeable paving system with plastic or stone can effectively remove heavy metals and the mechanism may be chemical sedimentation [
6]. Katherine et al. used limestone or zeolite to remove heavy metals from constructed wetlands with a removal efficiency of 98% [
7]. Some studies have shown that construction bricks have a good anchoring effect on heavy metals [
8], but the research is blurry with regards to the chemical form and content of heavy metals in the media, and the stability after fixing is worth exploring. There are also some studies to remove heavy metals from water using hazelnut and almond shells [
9], but these adsorbents are present in small quantities. In addition, there is little research on the existing form of heavy metals after being fixed by the percolation facility and after the environmental risk has been evaluated. Some studies have shown that the shape of the breakthrough curve is affected by the linear velocity, the concentration of metal ions in the solution, and the height of the bed [
10,
11,
12,
13,
14]. A study found that continuous column experiments can provide good references for environmental remediation applications such as infiltration facilities like permeable pavements [
15]. Various models such as the Adams-Bohart model and the Thomas model have been applied to analyze and interpret the experimental data and predict the effects of different process variables on the efficiency of the adsorption process [
16]. Chu et al. found that the Adams-Bohart model is essentially the same as the Thomas model [
17]. However, heavy metal removal by wastes bricks should be explored and the proper fitting models should be evaluated in order to better predict the removal behavior.
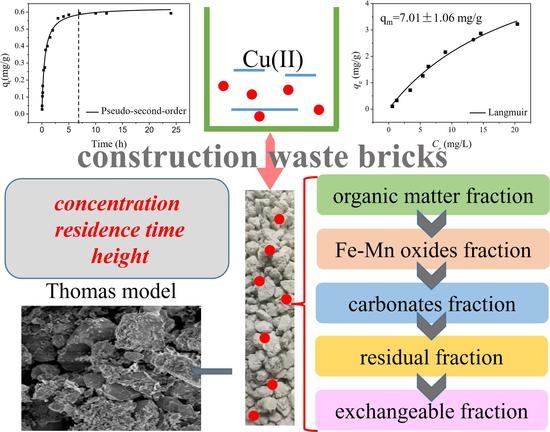
In the present study, the cost-effective lime sand bricks were selected as the adsorbent, their removal efficiency towards Cu(II) in aqueous solution was systematically studied through the static batch experiments and fixed bed continuous column experiments. The objectives of this study are (i) to study the adsorption mechanism of Cu(II) by lime sand bricks using batch experiments; (ii) to examine the effects of several process parameters such as the bed depth, solution concentration and residence time on removal efficiency of Cu(II); (iii) to analyze the effect of different process variables on the removal of Cu(II) by lime sand bricks and their applicability with the fitting model; (iv) to analyze the chemical forms of copper in the medium of lime sand bricks for the evaluation of the environmental risk.
2. Materials and Methods
2.1. Materials
Lime sand bricks were grounded before being used as adsorbents. Brick particles were then separated by sieving to achieve the particle size 2–5 mm. Then the samples were subjected to calcination in an oven at 105 °C for 24 h. The soaked brick particles were stirred in 1 mol/L sulfuric acid for 3 days to remove impurities from the brick surface and to prevent the excessive pH of the effluent leading to the precipitation of Cu(II). The acid washed bricks were then washed with pure water, dried, and set aside. The Cu(II) solution was prepared by using aqueous solutions of CuSO4·5H2O. Chemicals including NaOH, HNO3, and H2SO4 were purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China).
2.2. Characterization of Bricks
The brick particles were characterized using scanning electron microscopy (SEM, Hitachi Limited S-4800, Tokyo, Japan-SEM) to study the surface morphology. Brunauer–Emmett–Teller (BET, ASAP-2460, Norcross, GA, USA) surface area and Barrett–Joyner–Halenda (BJH) pore size and volume tests were performed on bricks to measure the surface area and porosity. The functional groups on brick were analyzed by Fourier transform infrared spectroscopy (FTIR, Bruker, Germany). The elemental composition of lime sand bricks was determined by X-ray fluorescence (XRF, Shimadzu XRF-1800, Kyoto, Japan).
2.3. Batch Absorption Experiments
The batch experiments were conducted to investigate the adsorption behavior of Cu(II) in lime sand bricks. The experiments were conducted at 25 °C. The adsorption kinetics was studied and adsorption isotherms were measured. All experiments were performed in duplicate. In the adsorption kinetics experiments, 500 mL solutions were prepared with a Cu(II) concentration of 5 mg/L. Brick powders of 2–5 mm were then added into the solution with a dosage of 8 g/L. Solution samples were taken at different time intervals and filtered with 0.45 μm pore diameter nylon micropore membranes. The concentration of Cu(II) in the filtrate was then analyzed by ICP-MS (Elan 5000, Perkin Elmer, Waltham, MA, USA). To measure the adsorption isotherms, a series of 40 mL solutions with a Cu(II) concentration of 0–30 mg/L were prepared. Adsorbents having the same size distribution were then added into the solutions with a dosage of 8 g/L. After reaching equilibrium, the solutions were filtered and the concentration of Cu(II) was analyzed. The pH was controlled using NaOH and HCl to ensure that the pH was around 5.
The data on the adsorption kinetics of Cu(II) were fitted using the pseudo-first-order (Equation (1)) and pseudo-second-order models (Equation (2)).
where
k1 (h
−1),
k2 (g/mg⋅h) are the rate constants of adsorption,
qe (mg/g) is the adsorption capacity at equilibrium and
qt is the amount of metal adsorbed at time
t (h).
Different models were used to correlate and compare experimental adsorption data to provide an accurate fit of the adsorption isotherms. In this study, the Freundlich and Langmuir isotherm models were used to fit the adsorption isotherms. The Freundlich and Langmuir models are shown in Equations (3) and (4).
where
qm (mg/g) is the maximum adsorption capacity of the materials;
KF((mg/g) (L/mg)
n) and
KL (L/mg) are the constants for the Freundlich and Langmuir models, respectively;
Ce (mg/L) is the metal concentration in solution at equilibrium;
n is a dimensionless empirical parameter, which gives information on the strength of the adsorption.
To investigate the effect of ionic strength on adsorption, 40 mL of a Cu(II) solution with an initial concentration of 5 mg/L was added to each centrifuge tube. The pH was controlled at 5 using NaOH and HCl. The lime sand bricks with a particle size of 0.5–1 mm were added to the solution, and the solid-liquid ratio was 8 g/L. Sodium chloride was used to adjust different levels of ionic strength (i.e., 0, 0.2, 1, 5, 10, 20, 50, 100 and 200 mg/L). All samples were shaken at 135 rpm.
2.4. Column Set-Up
The experimental setup is shown in
Figure 1. The experimental columns were employed to investigate the effects of inlet concentration, residence time, and heights on the removal of Cu(II). In order to simulate the use of lime sand bricks for the packing in the percolation facility, the plexiglass columns were employed with a size of 3 cm diameter and 20 cm height. The selected column height fits the design regulations of a permeable pavement by sponge city construction guide in China. In order to avoid sidewall effects as much as possible, the solution was injected from the bottom of the column using a peristaltic pump. Samples were taken at 5 cm, 10 cm, 15 cm, and 20 cm from the bottom of the column to investigate the removal of Cu(II) at different heights. Glass wool is added to each side of the columns to improve the flow rate of the water and prevent the loss of the adsorbent during the process. The quality of each pillar filled lime sand bricks was 195 g. The packing density was calculated to be 1.38 g/cm
3 based on the filling quality of the brick and the column size. The column porosity is achieved by controlling the flow rate of the peristaltic pump with a porosity of 50.03%. Water samples were collected from different outlets at different time intervals. The columns study was carried out at 25 °C. In order to ensure the exclusivity of the adsorption process, the pH of the metal solution was kept below the precipitation pH of the metal. Therefore, the pH of the initial solution was controlled to 2 by the addition of hydrochloric acid, which could ensure the pH of the effluent in the range of 5 to 6. The breakthrough curves and efficiency parameters for evaluating the adsorption performance of the fixed bed were obtained from the experimental data.
where
q (mg/g) is the adsorption capacity per unit mass of construction wastes at any one time;
qt (mg/g) is the adsorption capacity per unit mass of construction wastes;
V (L) is the volume of sample;
C0 (mg/L) is the concentration of inlet Cu(II);
Q (mL/min) is the inflow rate of the solution;
m (g) is the adsorbent quality;
Ct (mg/L) is the outlet concentration over a certain period of time.
2.5. The Effect of Bed Depth, Inlet Concentration and Residence Time
To evaluate the effect of bed depth, the height of the filled lime sand bricks was measured at 5, 10, 15 and 20 cm. A Cu(II) solution of 0.5 mg/L was added to the column at a flow rate corresponding to a residence time of 10 min. Samples were taken at different time intervals and filtered through a 0.45 μm membrane filter. A small amount of nitric acid was then added to the sample and stored in the fridge at 0–4 °C before analysis. The inlet concentration was determined according to the drinking water standards in China; the residence time and the height were designed based on the permeability of the permeable pavement and the height of the paving.
To examine the inlet concentration effect on the removal of Cu(II), the concentration of Cu(II) at 0.5, 1.0 and 2.0 mg/L were selected. The residence time of the influent was controlled at 10 min and the bed height was maintained at 20 cm. To study the effect of residence time on the removal of Cu(II), a residence time of 8, 10 and 12 min were selected. The inlet concentration of Cu(II) remained as 0.5 mg/L and the bed height was packed at 20 cm. The effluent was collected for metal analysis at different time intervals.
2.6. Response Analysis by Box–Behnken Design (BBD)
Response surface methodology (RSM) is a useful statistical tool for the optimization of different processes and widely used for experimental design. In this study, the optimization of experimental conditions for the removal of Cu(II) by lime sand bricks was conducted using Box–Behnken design (BBD) technique under RSM. In order to evaluate the removal effects of lime sand bricks on Cu(II), three main factors were chosen: height (cm) (X
1), concentration (mg/L) (X
2) and residence time (min) (X
3). The experimental range and levels of design are shown in
Table 1.
2.7. Adam–Bohart Model
The Adam–Bohart model describes the relationship between
Ct/
C0 and
t during continuous operation and is usually used to describe the inlet part of the breakthrough curve [
18]. The simplified form of the Adam–Bohart model used in this study is referred to as the bed depth service time (BDST) [
19]. The BDST model is used for the interpretation of the inlet part of the breakthrough curve that is up to 10–50% of the breakthrough. The BDST Equation (7) below shows a linear relationship between bed height and breakthrough time, often called service time at the bed.
where
t is the service time at the breakthrough point (hour);
N0 is the adsorption capacity per volume of the bed (mg/cm
3);
Z is the depth of the adsorbent bed (cm);
C0 is the influent or inlet solute concentration (mg/L);
θ is the linear flow rate (cm/h);
Ka is the rate constant of adsorption (L/mg·h) and
Cb is the effluent concentration at the breakthrough point (mg/L).
2.8. Analytical Methods of Cu(II) and its Sequential Extraction
The procedure proposed by Tessier et al. was applied in this study to analyzed different copper species that was usually used to determine the fractionation of heavy metals in soil and sediment samples [
20]. The Cu(II) of the five fractions were analyzed, including the exchangeable fraction, carbonates fraction, Fe-Mn oxides fraction (the fraction associated with Fe and Mn oxides), organic matter fraction (the fraction bound to organic matter) and a residual fraction. After the completion of the experimental column, lime sand bricks were taken out and the Cu(II) adsorbed to bricks were analyzed through sequential extraction in order to distinguish different species. The analysis was carried out for the brick flows through different inlet concentration of 0.5, 1.0 and 2.0 mg/L with a residence time of 10 min. The bricks were baked at 105 °C for 24 h until dry, ground with a mortar and then passed through a 200-mesh screen.
Step 1: exchangeable fraction (F1). The solid samples (2 g) were introduced into three 50 mL centrifuge tubes containing a 16 mL magnesium chloride solution (1 mol/L, adjusted to pH 7 with HNO3 and NaOH) and shaken for 1 h at 25 °C, respectively. The solution was separated from the solid by centrifugation at 4000 rpm for 20 min. The liquid supernatant was then filtered through a 0.45 μm membrane filter. The solid residue was retained for subsequent analysis.
Step 2: carbonates fraction (F2). A 16 mL volume of sodium acetate solution (1 mol/L, adjusted to pH 5 with HNO3) was added to the residue from step 1 in a centrifuge tube, which was shaken for 8 h at 25 °C. The solution was separated from the solid by centrifugation at 4000 rpm for 20 min. The extraction procedure depicted above was followed.
Step 3: Fe-Mn oxides fraction (F3). A 16 mL volume of hydroxylamine hydrochloride solution (0.04 mol/L, adjusted to pH 2 with HNO3) to 25% acetic acid solution in acetic acid solution was added to the residue from step 2, which was shaken for 4 h at 95 ± 2 °C. The solution was separated from the solid by centrifugation at 4000 rpm for 20 min. The extraction procedure depicted above was followed.
Step 4: organic matter fraction (F4). A 5 mL volume of hydrogen peroxide (30%) and 3 mL nitric acid (0.01 mol/L) were added to the residue from step 2. The mixture is heated to 85 °C in a water bath and kept at 85 °C for 2 h with occasional manual stirring. A second 5 mL volume of hydrogen peroxide (30%, adjusted to pH 2 with HNO3) was introduced and digested at 85 ± 1 °C for 2 h in a water bath. After that, 5 mL of 3.2 mol/L ammonium acetate (adjusted to pH 2 with HNO3) was introduced to the cooled-down residue, which was then shaken for 0.5 h at 25 °C. Finally, the extract was separated according to the procedure described in step 1.
Step 5: residual fraction (F5). We used the same method as determining the total concentration of heavy metals in the sample. The residue from the metal content of step 3 was digested with 10 mL of aquaregia heated on a heater and then evaporated to near dryness according to ISO recommendations. After cooling down, the residues were diluted in 50 mL 5% HNO3.
In order to determine the total concentration of Cu(II) in the top layer of the lime sand bricks in the column, the lime sand bricks were used for digestion by microwave digestion.
A total of 0.1 g of brick sample was weighed into a poly tetra fluoro ethylene digestion tank, and a blank sample of 0.1 mL of ultrapure water was used instead of the brick sample in one digestion tank. A total of 5 mL volume of nitric acid, 2 mL volume of hydrogen peroxide and 3 mL volume of hydrofluoric acid were sequentially added to the digestion tank. After cooling, the solution was transferred to a polytetrafluoroethylene crucible, and heated at 130 °C on a hot plate to catch the acid to a viscous state. After removing the crucible, the samples were transferred to 100 mL volumetric flask and dilute to the mark with water.
The solution concentrations before and after the adsorption process were determined by ICP-MS.
4. Conclusions
The lime sand bricks were systematically investigated as potential filter materials for the removal of Cu(II) from aqueous solutions using static batch experiments and fixed bed continuous column experiments. The study provides basic knowledge on using construction wastes bricks as a potential economical medium for removing heavy metals in the aqueous phase. The conclusions are listed as follows:
(1) The adsorption process revealed that the initial uptake was achieved in 7 h. The isotherm results showed that the amount of Cu(II) adsorbed is large and the maximum adsorption capacity could reach 7.01 mg/g.
(2) Higher bed depths (20 cm) and a longer residence time (12 min) contributed to a more efficient treatment system in the fixed-bed column.
(3) The adsorption capacity of lime sand bricks increased with the increase of inlet concentration. When the inlet concentration was 2.0 mg/L, the adsorption capacity reached at 169.59 mg/kg.
(4) The Box–Behnken design results show that the inlet concentration and residence time in the column experiment have significant effects on the removal of Cu(II) by lime sand bricks. The Adams-Bohart model with a high r2 (0.99) can predict the change of the effluent concentration with time in the Cu(II) removal process.
(5) The ecotoxicity, bioavailability and mobility of Cu(II) in lime sand bricks depended on their chemical speciation rather than their total concentrations. Sequential extraction showed that the lime sand bricks after adsorption of copper can decrease the ecotoxicity and bioavailability.