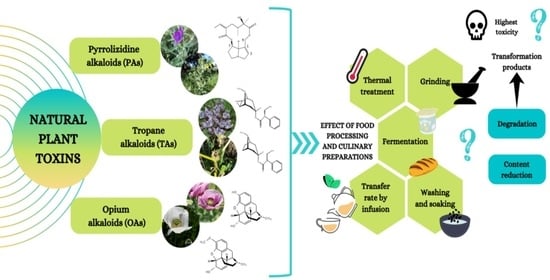
Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants
Abstract
:1. Introduction
2. Natural Toxic Plant Alkaloids with Relevant Occurrence in Food
2.1. Origin, Toxicity and Occurrence of Pyrrolizidine Alkaloids (PAs)

2.2. Origin, Toxicity and Occurrence of Tropane Alkaloids (TAs)
2.3. Origin, Toxicity and Occurrence of Opium Alkaloids (OAs)
3. Effect of Food Processing and Culinary Preparations on PAs, TAs and OAs
3.1. Thermal Treatment
3.2. Fermentation
| Analytes | Sample | Thermal Treatment Conditions | Effect Observed | Reference |
|---|---|---|---|---|
| Pyrrolizidine alkaloids | ||||
| Re, Jb, JbNO, Jl, Jz, DehydroJc, HydroxyJb, Eruc, Sk, Ot, Fl | Thermized milk | Pasteurization: 76 °C for 15 s Sterilization: 140 °C for 4 s | No significant effects observed | [67] |
| Re | Maize flour and maize porridge | Heating in a boiling water bath for 3 h | Approximately reduction of 40% | [71] |
| Re, Sc, Jb, Sp, Im, He, Eu, Sk, ReNO, ScNO, JbNO, SpNO, ImNO, HeNO, EuNO | Green and black teas | Black tea: dried at 110 °C Green tea: fixed at 220–230 °C for de-enzyme and dried at 110 °C | Reduction of 0.8–79.2% | [72] |
| He, HeNO, Ls, LsNO, Sc, ScNO, Ig, IgNO, Sa | Pollen | Heating at 35, 40 and 60 °C for 72 and 84 h. | Reduction of 30–75% | [75] |
| Re, Ech, Im, ImNO, Sc, EchNo, ReNO, Ev | Bee bread | 30 °C for 6 months | Reduction of 33–39% | [77] |
| Tropane alkaloids | ||||
| At | Datura stramonium and Brugmansia seeds and buckwheat | Heating at 80 °C and pH 9 | Racemization of At | [41] |
| At, Scp | Bread crumb a Bread bark a | Baking at 215 °C for 35 min | Bread crumb: Reduction of 13–25% Bread bark: Reduction of 18–28% | [78] |
| An, Scp, At, Lt, Ap, Hm, Apo, Scpl, Tropi | Bread b | Baking at 190 °C for 40 min | Reduction of 11–100%, except Apo and Ap which increased, possibly due to the degradation and transformation of other analytes into these compounds | [79] |
| At, Scp, An | Breadsticks | Baking at 180 °C for 20 min | Reduction of 7–65% | [80] |
| An, Scp, At, Lt, Ap, Hm, Apo, Scpl, Tropi | Pasta | Boiling at 100 °C for 10 min | Reduction of 24–66% | [81] |
| An, Scp, Coc, Benzoylec, At, Lt, Ap, Apo, Hm, Ch, Ecg, Scpl, Tropi, Trpn, Tr | Tea | Water infusion at 100 °C, left to cool for 5 min | Reduction of 2–87%, except Apo, Ap, Ecg and Tropi, which increased, possibly due to the degradation and transformation of other analytes into these compounds | [81] |
| Opium alkaloids | ||||
| Mor, Cod | Cakes Buns | Cakes: Baking at 180 °C for 20 min Buns: Baking at 220 °C for 20 min (toppings) | Cakes: Reduction of 50–90% Buns: Reduction of 93–97% | [52] |
| Mor, Cod, Th, Pap, Nos | Muffins and Rolls | Baking at 180 °C for 15 min | Reduction of 100% | [56] |
| Mor, Cod, Th, Pap, Nos | Breadsticks | Baking at 180 °C for 20 min | Reduction of 23–100% | [80] |
| Mor | Bread | Conditions #1: Baking at 135 °C Conditions #2: Baking at 220 °C | Conditions #1: Reduction of 10–50% Conditions #2: Reduction pf 30% | [82] |
| Mor, Cod, Th | Muffin | Baking at 120 °C for 120 min | No significant effects observed | [83] |
| Mor | Cake | Baking at 180 °C for 20 min | Reduction of 55–75% | [85] |
| Analytes | Sample | Fermentation Conditions | Fermentation Effect | Reference |
|---|---|---|---|---|
| Pyrrolizidine alkaloids | ||||
| Re, Jb, JbNO, Jl, Jz, DehydroJc, HydroxyJb, Eruc, Sk, Ot, Fl | Yoghurt | 0.02% v/v starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus) 42 °C for 6 h until pH 4.4 | Reduction of 27% | [67] |
| Re, Jb, JbNO, Jl, Jz, DehydroJc, HydroxyJb, Eruc, Sk, Ot, Fl | Cheese | 0.04% v/v mixed-strain mesophilic starter culture (composed of various strains of the species Lactococcus lactis cremoris and Leuconostoc spp.), Rennet of animal origin, 31 °C for 56 min | Reduction of 14% | [67] |
| Re, Sc, Jb, Sp, Im, He, Eu, Sk, ReNO, JbNO, SpNO, ScNO, ImNO, HeNO, EuNO | Black tea | Under high relative humidity and temperature for 5–6 h | Increase in the PA and PANO content | [72] |
| n.p. *a | Mead | n.p. * | Values found well above the average of regular retail honey | [86] |
| Ech, EchNO, Echi, EchiNO, AcetylEch, AcetylEchNO, AcetylEchi, AcetylEchiNO | Mead | n.p. * | Reduction of 30–70% | [87] |
| Tropane alkaloids | ||||
| An, Scp, At, Lt, Ap, Hm, Apo, Scpl, Tropi | Bread | 37 °C during 1 h | Reduction of 19–65% (except for Ap, Apo and Tropi) b | [79] |
3.3. Infusion
| Analytes | Sample | Brewing Conditions | Transfer Rate | Reference |
|---|---|---|---|---|
| Pyrrolizidine alkaloids | ||||
| Ech, EchNO, En, EnNO, Eu, EuNO, Hs, HsNO, He, HeNO, In, InNO, Ig, IgNO, Im, ImNO, Ls, LsNO, Lyc, LycNO, Re, ReNO, Sc, ScNO, Sp, SpNO, Sv, SvNO, Sk, Us | Fennel and anise teas | n.p. * | n.p. * (lower than 100%) a | [70] |
| Re | Lucerne herbal tea | 100 °C for 3 h | Approximately 100% | [71] |
| Re, Sc, Jb, Sp, Im, He, Eu, Sk, ReNO, ScNO, JbNO, SpNO, ImNO, HeNO, EuNO | Green tea | First infusion: 100 °C for 4 min Second infusion: 100 °C for 4 min | 41–93% | [72] |
| Ech, Eruc, Eu, He, In, Im, Jb, Ls, Lyc, Mc, Re, Sc, Sp, Sv, Sk, Td, EchNO, ErucNO, EuNO, HeNO, InNO, ImNO, JbNO, LsNO, LycNO, McNO, ReNO, ScNO, SpNO, SvNO | Rooibos tea | 100 °C for 6 min | 16–45% | [90] |
| Ech, Lyc, Mc, Sc, ScNO, Sp, He, Ls, Sk, Re | Herbal teas (mix, rooibos, linden, mint, verbena, camomile) | 100 °C for 10 min with slight agitation | Approximately 100% | [93] |
| Sk | Coltsfoot (Tussilago farfara L.) | n.p. * | 80% | [94] |
| Re, Ls, LsNO, He, HeNO, Td, Mc, Sc, Sp, Ech, EchNO, Eruc, ErucNO, Eu, EuNO, Im, ImNO, Jb, JbNO, Lyc, LycNO, McNO, ReNO, ScNO, SpNO, Sv, SvNO, Sk | Teas (black tea, green tea) and herbal teas (rooibos, chamomile, peppermint, mix) | 100 °C for 5 min | Overall, 85% (individual samples varied between 40 and 250%) | [95] |
| 9 PAs | Peppermint herbal tea | n.p. * | 84–103% | [96] |
| Ech, Eruc, ErucNO, Eu, EuNO, He, HeNO, Im, Jb, JbNO, Ls, LsNO, Lyc, Mc, McNO, Re, ReNO, Sc, ScNO, Sp, SpNO, Sk, Td | Teas and herbal teas (nettle, fennel fruits, chamomile, melissa, peppermint, mix (valerian, hopcone, lavender, caraway, anise, coriander)) | 100 °C for 15 min | 1–21% | [97] |
| Ech, ErucNO, Eu, EuNO, Im, ImNO, Jb, JbNO, Ls, Lyc, LycNO, Mc, Sc, ScNO, Sk, Sv, SvNO, Td, Re, Sp, Jl, Hs, Rn, Ev, EchNO, HeNO, McNO, LsNO, ReNO, SpNO | Black tea, green tea, mixed herbal tea, peppermint tea, red bush tea, senna tea | ISO brewing procedure: 100 °C for 6 min (capping and shaking). The procedure was repeated two more times. Vendor’s instructions for brewing: - Black tea: 100 °C for 4 min (uncapped) - Green tea: first extraction at 80 °C for 2 min (uncapped) + second extraction at 100 °C for 1 min (uncapped) - Mixed herbal tea: 100 °C for 10 min (capped) - Peppermint tea: 100 °C for 6 min (uncapped) - Red bush tea: 100 °C for 6 min (uncapped) - Senna tea: 80 °C for 10 min (uncapped) | 52–100% (ISO brewing procedure) 63–100% (vendor’s instructions) | [98] |
| Tropane alkaloids | ||||
| At, Scp | Tea | 100 °C for 4.5 min | 42–54% | [35] |
| An, Scp, At, Lt, Ap, Hm, Apo, Scpl, Tropi | Tea | Tea: 100 °C, left to cool for 5 min | 20–92% | [81] |
| At, Scp | Fennel tea | 100 °C for 5 min | 47–88% | [101] |
| Opium alkaloids | ||||
| Morphine (Mor), Codeine (Cod), Thebaine (Th) Papaverine (Pap), Noscapine (Nos) | Poppy seed tea | 90 °C for 5 min | 71–100% | [104] |
3.4. Other Treatments (Grinding, Washing and Soaking)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs. Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 18 October 2022).
- European Commission. Food Safety, Farm to Fork Strategy. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 18 October 2022).
- Ridgway, K.; Lalljie, S.P.; Smith, R.M. Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef]
- AESAN. Agencia Española de Seguridad Alimentaria y Nutrición. Available online: https://www.aesan.gob.es (accessed on 18 October 2022).
- EFSA. European Food Safety Authority. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/contaminants_in_the_food_chain.pdf (accessed on 18 October 2022).
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Casado-Hidalgo, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Opium Alkaloids in Food Products: Current and Future Perspectives. Trends Food Sci. Technol. 2021, 108, 92–102. [Google Scholar] [CrossRef]
- RASFF. Food and Feed Safety Alerts. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 19 October 2022).
- European Union. Commission Regulation (EU) 2020/2040 of 11 December 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of pyrrolizidine alkaloids in certain foodstuffs (Text with EEA relevance) (OJ L 420 14.12.2020, p. 1). Available online: http://data.europa.eu/eli/reg/2020/2040/oj (accessed on 22 January 2023).
- European Union. Commission regulation (EU) 2021/1408 of 27 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of tropane alkaloids in certain foodstuffs. Off. J. Eur. Union 2021, 1–4. [Google Scholar]
- European Union. Commission Regulation (EU) 2021/2142 of 3 December 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of opium alkaloids in certain foodstuffs (Text with EEA relevance) (OJ L 433 06.12.2021, p. 8). Available online: http://data.europa.eu/eli/reg/2021/2142/oj (accessed on 22 January 2023).
- Kaltner, F. Fate of Food-Relevant Toxic Plant Alkaloids during Food Processing or Storing and Analytical Strategies to Unveil Potential Transformation Products. J. Agric. Food Chem. 2022, 70, 5975–5981. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New advanced materials and sorbent-based microextraction techniques as strategies in sample preparation to improve the determination of natural toxins in food samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Natural Toxins in Food. Available online: https://www.who.int/news-room/fact-sheets/detail/natural-toxins-in-food (accessed on 19 October 2022).
- European Commission. Food Safety. Available online: https://food.ec.europa.eu/safety/chemical-safety/contaminants/catalogue/plant-toxins_en (accessed on 19 October 2022).
- Nollet, L.M.L.; Ahmad, J. Naturally Occurring Food Toxins—An Overview (Chapter 1). In Analysis of Naturally Occurring Food Toxins of Plant Origin, 1st ed.; Nollet, L.M.L., Ahmad, J., Eds.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Dusemund, B.; Schaefer, B.; Lampen, A. Plant Alkaloids. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 344–347. [Google Scholar] [CrossRef]
- EFSA-European Food Safety Authority. Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Keuth, O.; Humpf, H.U.; Fürst, P. Pyrrolizidine Alkaloids: Analytical Challenges. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 348–355. [Google Scholar] [CrossRef]
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.M.C.M.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment—A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Beuerle, T. Toxic pyrrolizidine alkaloids in herbal medicines commonly used in Ghana. J. Ethnopharmacol. 2017, 202, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Wittke, C.; Lederer, I.; Klier, B.; Kleinwächter, M.; Selmar, D. Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chem. 2016, 213, 163–168. [Google Scholar] [CrossRef]
- Selmar, D.; Radwan, A.; Nowak, M. Horizontal natural product transfer: A so far unconsidered source of contamination of plant-derived commodities. J. Environ. Anal. Toxicol. 2015, 5, 1000287. [Google Scholar] [CrossRef]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pol. 2019, 248, 456–461. [Google Scholar] [CrossRef]
- Chmit, M.S.; Horn, G.; Dübecke, A.; Beuerle, T. Pyrrolizidine Alkaloids in the Food Chain: Is Horizontal Transfer of Natural Products of Relevance? Foods 2021, 10, 1827. [Google Scholar] [CrossRef]
- Letsyo, E.; Adams, Z.S.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2021, 270, 128669. [Google Scholar] [CrossRef]
- Jiao, W.; Shen, T.; Wang, L.; Zhu, L.; Li, Q.X.; Wang, C.; Chen, H.; Huam, R.; Wu, X. Source and Route of Pyrrolizidine Alkaloid Contamination in Tea Samples. JOVE 2022, 187. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Tropane alkaloids in food and feed. EFSA J. 2013, 11, 3386. [Google Scholar] [CrossRef]
- Eich, E. Solanaceae and Convolvulaceae: Secondary Metabolites Biosynthesis, Chemotaxonomy, Biological and Economic Significance (A Handbook); Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74541-9. [Google Scholar]
- Cirlini, M.; Cappucci, V.; Galaverna, G.; Dall’Asta, C.; Bruni, R. A sensitive UHPLC-ESI-MS/MS method for the determination of tropane alkaloids in herbal teas and extracts. Food Control 2019, 105, 285–291. [Google Scholar] [CrossRef]
- Gonçalves, C.; Cubero-Leon, E.; Stroka, J. Determination of tropane alkaloids in cereals, tea and herbal infusions: Exploiting proficiency testing data as a basis to derive interlaboratory performance characteristics of an improved LC-MS/MS method. Food Chem. 2020, 331, 127260. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; de Nijs, M.; Castellari, M.; Hortos, M.; MacDonald, S.; Crews, C.; Hajslova, J.; Stranska, M. Occurrence of tropane alkaloids in food. EFSA Support. Publ. 2016, 13, 1140E. [Google Scholar] [CrossRef]
- Todd, F.G.; Stermitz, F.R.; Schultheis, P.; Knight, A.P.; Traub-Dargatz, J. Tropane alkaloids and toxicity of Convolvulus arvensis. Phytochemistry 1995, 39, 301–303. [Google Scholar] [CrossRef]
- Abia, W.A.; Montgomery, H.; Nugent, A.P.; Elliott, C.T. Tropane alkaloid contamination of agricultural commodities and food products in relation to consumer health: Learnings from the 2019 Uganda food aid outbreak. Compr. Rev. Food Sci. Food Saf. 2021, 20, 501–525. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, L.; Morante-zarcero, S.; Pereira, J.A.M.; Câmara, J.S.; Sierra, I. Improved Analytical Approach for Determination of Tropane Alkaloids in Leafy Vegetables Based on µ -QuEChERS Combined with HPLC-MS/MS. Toxins 2022, 14, 650. [Google Scholar] [CrossRef]
- European Commission. Commision Recommendation (EU) 2015/976 of 19 June 2015 on the monitoring of the presence of tropane alkaloids in food. Off. J. Eur. Union 2015, 11, 97–98. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2016/239 of 19 February 2016 amending Regulation (EC) No 1881/2006 as regards maximum levels of tropane alkaloids in certain cereal-based foods for infants and young children. Off. J. Eur. Union 2016, L45, 3–5. [Google Scholar]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Enantiomeric determination and evaluation of the racemization process of atropine in Solanaceae seeds and contaminated samples by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1474, 79–84. [Google Scholar] [CrossRef] [Green Version]
- González-Gómez, L.; Gañán, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Sulfonic Acid-Functionalized SBA-15 as Strong Cation-Exchange Sorbent for Solid-Phase Extraction of Atropine and Scopolamine in Gluten-Free Grains and Flours. Foods 2020, 9, 1854. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, L.; Pereira, J.A.M.; Morante-Zarcero, S.; Câmara, J.S.; Sierra, I. Green extraction approach based on μSPEed® followed by HPLC-MS/MS for the determination of atropine and scopolamine in tea and herbal tea infusions. Food Chem. 2022, 394, 133512. [Google Scholar] [CrossRef]
- Romera-Torres, A.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Simultaneous analysis of tropane alkaloids in teas and herbal teas by liquid chromatography coupled to high-resolution mass spectrometry (Orbitrap). J. Sep. Sci. 2018, 41, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Mesostructured Silicas as Cation-Exchange Sorbents in Packed or Dispersive Solid Phase Extraction for the Determination of Tropane Alkaloids in Culinary Aromatics Herbs by HPLC-MS/MS. Toxins 2022, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Fernández, D.; Moreno-González, D.; García-Reyes, J.F.; Ballesteros, E.; Molina-Díaz, A. Determination of atropine and scopolamine in spinach-based products contaminated with genus Datura by UHPLC–MS/MS. Food Chem. 2021, 347, 129020. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.J.; Pereboom-de Fauw, D.P.K.H.; Hoogenboom, R.L.A.P.; de Stoppelaar, J.; de Nijs, M. Tropane and ergot alkaloids in grain-based products for infants and young children in the Netherlands in 2011–2014. Food Addit. Contam. Part B Surveill. 2015, 8, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Demuth, T.M.; Biancardi, A.; Rychlik, M.; Dall’Asta, C.; Bruni, R. Are tropane alkaloids present in organic foods? Detection of scopolamine and atropine in organic buckwheat (Fagopyron esculentum L.) products by UHPLC–MS/MS. Food Chem. 2018, 239, 141–147. [Google Scholar] [CrossRef]
- EFSA. Update of the Scientific Opinion on Opium Alkaloids in Poppy Seeds. EFSA J. 2018, 16, e05243. [Google Scholar] [CrossRef]
- AESAN (Spanish Food Safety and Nutrition Agency). Opium Alkaloids in Poppy Seeds. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_cc_ingles/POPPY_SEEDS.pdf (accessed on 15 October 2022).
- López, P.; Pereboom-de Fauw, D.P.K.H.; Mulder, P.P.J.; Spanjer, M.; de Stoppelaar, J.; Mol, H.G.J.; de Nijs, M. Straightforward Analytical Method to Determine Opium Alkaloids in Poppy Seeds and Bakery Products. Food Chem. 2018, 242, 443–450. [Google Scholar] [CrossRef]
- Sproll, C.; Perz, R.C.; Lachenmeier, D.W. Optimized LC/MS/MS Analysis of Morphine and Codeine in Poppy Seed and Evaluation of Their Fate during Food Processing as a Basis for Risk Analysis. J. Agric. Food Chem. 2006, 54, 5292–5298. [Google Scholar] [CrossRef]
- Powers, D.; Erickson, S.; Swortwood, M.J. Quantification of Morphine, Codeine, and Thebaine in Home-Brewed Poppy Seed Tea by LC-MS/MS. J. Forensic Sci. 2018, 63, 1229–1235. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Mesostructured Silica-Coated Magnetic Nanoparticles to Extract Six Opium Alkaloids in Poppy Seeds Prior to Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Analysis. Foods 2021, 10, 1587. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Martínez-García, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. New Validated Method for the Determination of Six Opium Alkaloids in Poppy Seed-Containing Bakery Products by High-Performance Liquid Chromatography-Tandem Mass Spectrometry after Magnetic Solid-Phase Extraction. J. Agric. Food Chem. 2022, 70, 7594–7606. [Google Scholar] [CrossRef]
- Carlin, M.G.; Dean, J.R.; Ames, J.M. Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2020, 8, 737. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Pulsed Ultrasound-Assisted Extraction Followed by Purification with SBA-15 for the Control of Opium Alkaloids in Biscuits and Sponge Cakes. Microchem. J. 2022, 183, 108059. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Zhu, L.; Ji, H.; Song, X.; Guo, H.; Yi, T. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem. Toxicol. 2018, 119, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Mandić, B.M.; Vlajić, M.D.; Trifunović, S.S.; Simić, M.R.; Vujisić, L.V.; VuČković, I.M.; Novaković, M.M.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; et al. Optimisation of isolation procedure for pyrrolizidine alkaloids from Rindera umbellata Bunge. Natural Prod. Res. 2015, 29, 887–890. [Google Scholar] [CrossRef]
- Mroczek, T.; Ndjoko-Ioset, K.; Głowniak, K.; Miętkiewicz-Capała, A.; Hostettmann, K. Investigation of Symphytum cordatum alkaloids by liquid–liquid partitioning, thin-layer chromatography and liquid chromatography–ion-trap mass spectrometry. Anal. Chim. Acta 2006, 566, 157–166. [Google Scholar] [CrossRef]
- Qi, X.; Wu, B.; Cheng, Y.; Qu, H. Simultaneous characterization of pyrrolizidine alkaloids and N-oxides in Gynura segetum by liquid chromatography/ion trap mass spectrometry. Rapid Comm. Mass Spectro. 2009, 23, 291–302. [Google Scholar] [CrossRef]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.F.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in Western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J. Toxicol. 2010, 2010, 313280. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam. Part A 2011, 28, 293–307. [Google Scholar] [CrossRef]
- Willmot, F.C.; Robertson, G.W. Senecio disease, or cirrhosis of the liver, due to Senecio poisoning. Lancet 1920, 196, 848–849. [Google Scholar] [CrossRef]
- Mohabbat, O.; Younos, M.S.; Merzad, A.A.; Srivastava, R.N.; Sediq, G.G.; Aram, G.N. An outbreak of hepatic veno-occlusive disease in north-western Afghanistan. Lancet 1976, 308, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.K.; Fakhrutdinova, S.S. Assessment of meat and milk in cases of Trichodesma poisoning. Vet. Mosc. USSR 1971, 8, 104–105. [Google Scholar]
- De Nijs, M.; Mulder, P.P.; Klijnstra, M.D.; Driehuis, F.; Hoogenboom, R.L. Fate of pyrrolizidine alkaloids during processing of milk of cows treated with ragwort. Food Addit. Contam. Part A 2017, 34, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Klein, L.M.; Gabler, A.M.; Rychlik, M.; Gottschalk, C.; Kaltner, F. A sensitive LC–MS/MS method for isomer separation and quantitative determination of 51 pyrrolizidine alkaloids and two tropane alkaloids in cow’s milk. Anal. Bioanal. Chem. 2022, 414, 8107–8124. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of pyrrolizidine alkaloids in food. EFSA Support. Pub. 2015, 12, 859E. [Google Scholar] [CrossRef]
- Jansons, M.; Fedorenko, D.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Nanoflow liquid chromatography mass spectrometry method for quantitative analysis and target ion screening of pyrrolizidine alkaloids in honey, tea, herbal tinctures, and milk. J. Chromatogr. A 2022, 1676, 463269. [Google Scholar] [CrossRef]
- Rosemann, G.M. Analysis of pyrrolizidine alkaloids in Crotalaria species by HPLCMS/MS in order to evaluate related food health risks Thesis. Ph.D. Thesis, University of Pretoria, Hatfield, Pretoria, 2006. [Google Scholar]
- Han, H.; Jiang, C.; Wang, C.; Lu, Y.; Wang, Z.; Chai, Y.; Zhang, X.; Liu, X.; Lu, C.; Chen, H. Dissipation pattern and conversion of pyrrolizidine alkaloids (PAs) and pyrrolizidine alkaloid N-oxides (PANOs) during tea manufacturing and brewing. Food Chem. 2022, 2022, 133183. [Google Scholar] [CrossRef]
- Hösch, G.; Wiedenfeld, H.; Dingermann, T.; Röder, E. A new high performance liquid chromatography method for the simultaneous quantitative analysis of pyrrolizidine alkaloids and their N-oxides in plant material. Phytochem. Anal. 1996, 7, 284–288. [Google Scholar] [CrossRef]
- Mattocks, A.R. Chemistry and Toxicology of Pyrrolizidine Alkaloids; Academic Press: London, UK, 1986. [Google Scholar]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Influence of storage on the stability of toxic pyrrolizidine alkaloids and their N-oxides in peppermint tea, hay, and honey. J. Agric. Food Chem. 2018, 66, 5221–5228. [Google Scholar] [CrossRef]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Bieri, K.; Zoller, O. Pyrrolizidine alkaloids: The botanical origin of pollen collected during the flowering period of Echium vulgare and the stability of pyrrolizidine alkaloids in bee bread. Molecules 2019, 24, 2214. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Composition of Jimson Weed (Datura stramonium) Seeds. J. Agric. Food Chem. 1989, 37, 998–1005. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Degradation of tropane alkaloids in baked bread samples contaminated with Solanaceae seeds. Food Res. Int. 2019, 122, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Vera-Baquero, F.L.; Morante-Zarcero, S.; Sierra, I. Evaluation of Thermal Degradation of Tropane and Opium Alkaloids in Gluten-Free Corn Breadsticks Samples Contaminated with Stramonium Seeds and Baked with Poppy Seeds under Different Conditions. Foods 2022, 11, 2196. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Effect of tea making and boiling processes on the degradation of tropane alkaloids in tea and pasta samples contaminated with Solanaceae seeds and coca leaf. Food Chem. 2019, 287, 265–272. [Google Scholar] [CrossRef]
- European Commission. 2014/662/EU: Commission Recommendation of 10 September 2014 on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products Text with EEA relevance (OJ L 271 12.09.2014, p. 96). Available online: http://data.europa.eu/eli/reco/2014/662/oj (accessed on 22 January 2023).
- Shetge, S.A.; Dzakovich, M.P.; Cooperstone, J.L.; Kleinmeier, D.; Redan, B.W. Concentrations of the Opium Alkaloids Morphine, Codeine, and Thebaine in Poppy Seeds are Reduced after Thermal and Washing Treatments but are Not Affected when Incorporated in a Model Baked Product. J. Agric. Food Chem. 2020, 68, 5241–5248. [Google Scholar] [CrossRef] [PubMed]
- Kleinmeier, D.; Pettengill, E.; Redan, B.W. Commentary: Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2021, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, M.; Golombek, P.; Lachenmeier, D.W. Reduction of Morphine During Baking? Response: Commentary: Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2021, 9, 692045. [Google Scholar] [CrossRef]
- Kempf, M.; Wittig, M.; Schönfeld, K.; Cramer, L.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in food: Downstream contamination in the food chain caused by honey and pollen. Food Addit. Contam. Part A 2011, 28, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Colegate, S.M.; Edgar, J.A. Persistence of echimidine, a hepatotoxic pyrrolizidine alkaloid, from honey into mead. J. Food Comp. Anal. 2013, 29, 106–109. [Google Scholar] [CrossRef]
- Casado, N.; Fernández-Pintor, B.; Morante-Zarcero, S.; Sierra, I. Quick and Green Microextraction of Pyrrolizidine Alkaloids from Infusions of Mallow, Calendula, and Hibiscus Flowers Using Ultrahigh-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry Analysis. J. Agric. Food Chem. 2022, 70, 7826–7841. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. Determination and occurrence of alkenylbenzenes, pyrrolizidine and tropane alkaloids in spices, herbs, teas, and other plant-derived food products using chromatographic methods: Review from 2010–2020. Food Rev. Int. 2021, 1–27. [Google Scholar] [CrossRef]
- Picron, J.F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chem. 2018, 266, 514–523. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Dietary exposure assessment to pyrrolizidine alkaloids in the European population. EFSA J. 2016, 14, 4572. [Google Scholar] [CrossRef] [Green Version]
- EFSA, European Food Safety Authority. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar] [CrossRef] [Green Version]
- Mathon, C.; Edder, P.; Bieri, S.; Christen, P. Survey of pyrrolizidine alkaloids in teas and herbal teas on the Swiss market using HPLC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 7345–7354. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, J.; Zeifel, U.; Schlatter, C.; Benn, M.H. Pyrrolizidine- Alkaloide in Huflattich (Tussilago farfara L.) verschiedener Herkunft [Pyrrolizidine alkaloids in coltsfoot (Tussilago farfara L.) of diverse origins]. Mitt Gebiete Lebensm Hyg. 1980, 71, 73–80. [Google Scholar]
- Mulder, P.P.J.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss- Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Engeli, B. Pyrrolizidine alkaloids in herbal teas, tea, iced tea beverages and aromatic herbs. In Proceedings of the Oral Presentation at: German Senate Commission on Food Safety SKLM, Hannover, Germany, 7 May 2014. [Google Scholar]
- Schulz, M.; Meins, J.; Diemert, S.; Zagermann-Muncke, P.; Goebel, R.; Schrenk, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Detection of pyrrolizidine alkaloids in German licensed herbal medicinal teas. Phytomedicine 2015, 22, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, H.; Zoller, O. Pyrrolizidine alkaloids in tea, herbal tea and iced tea beverages–survey and transfer rates. Food Addit. Contam. Part A 2021, 38, 1914–1933. [Google Scholar] [CrossRef]
- ISO standard 3103:1980; Tea—Preparation of Liquor for Use in Sensory Tests. International Organisation for Standardisation, 1980. Available online: https://www.iso.org/standard/8250.html (accessed on 22 January 2023).
- Chen, L.; Mulder, P.P.; Peijnenburg, A.; Rietjens, I.M. Risk assessment of intake of pyrrolizidine alkaloids from herbal teas and medicines following realistic exposure scenarios. Food Chem. Toxicol. 2019, 130, 142–153. [Google Scholar] [CrossRef]
- González-Gómez, L.; Gañán, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Atropine and scopolamine occurrence in spices and fennel infusions. Food Control 2023, 146, 109555–109563. [Google Scholar] [CrossRef]
- Li, S.Y.; Swortwood, M.J.; Yu, J.; Chi, C. Determination of morphine, codeine, and thebaine concentrations from poppy seed tea using magnetic carbon nanotubes facilitated dispersive micro-solid phase extraction and GC-MS analysis. Forensic Sci. Int. 2021, 329, 111052. [Google Scholar] [CrossRef]
- Montgomery, M.T.; Conlan, X.A.; Barnett, N.W.; Theakstone, A.G.; Quayle, K.; Smith, Z.M. Determination of morphine in culinary poppy seed tea extractions using high performance liquid chromatography with chemiluminescence detection. Aust. J. Forensic Sci. 2019, 51, S225–S228. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Perestelo, R.; Morante-Zarcero, S.; Cámara, J.S.; Sierra, I. Evaluation of the transfer and occurrence of opium alkaloids in poppy seed tea by a preconcentration with µ-SPEed followed by GC-MS analysis. Artic. Revis. 2023, 11, 94. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, N.; Casado-Hidalgo, G.; González-Gómez, L.; Morante-Zarcero, S.; Sierra, I. Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants. Appl. Sci. 2023, 13, 1704. https://doi.org/10.3390/app13031704
Casado N, Casado-Hidalgo G, González-Gómez L, Morante-Zarcero S, Sierra I. Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants. Applied Sciences. 2023; 13(3):1704. https://doi.org/10.3390/app13031704
Chicago/Turabian StyleCasado, Natalia, Gema Casado-Hidalgo, Lorena González-Gómez, Sonia Morante-Zarcero, and Isabel Sierra. 2023. "Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants" Applied Sciences 13, no. 3: 1704. https://doi.org/10.3390/app13031704