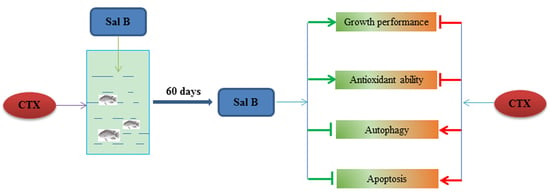
Salvianolic Acid B Regulates Oxidative Stress, Autophagy and Apoptosis against Cyclophosphamide-Induced Hepatic Injury in Nile Tilapia (Oreochromis niloticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Determination of Growth Indicators
2.3. Histopathological Observations
2.4. TUNEL Assay
2.5. Determination of Biochemical Indicators
2.6. Determination of mRNA Level
2.7. Statistical Analysis
3. Results
3.1. Effects of Sal B on Growth Performance of Nile Tilapia
3.2. Effects of Sal B on the Histopathology of Nile Tilapia
3.3. Effects of Sal B on TUNEL Assay
3.4. Effects of Sal B on Biochemical Indexes of Nile Tilapia
3.5. Effects of Sal B on mRNA Levels of Antioxidant-Related Genes in Nile Tilapia
3.6. Effects of Sal B on mRNA Levels of Autophagy-Related Genes in Nile Tilapia
3.7. Effects of Sal B on mRNA Levels of Apoptosis-Related Genes in Nile Tilapia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, H.; Pluta, H.J.; Braunbeck, T. Sublethal effects of prolonged exposure to disulfoton in rainbow trout (Oncorhynchus mykiss): Cytological alterations in the liver by a potent acetylcholine esterase inhibitor. Ecotoxicol. Environ. Saf. 1996, 34, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Moutou, K.; Braunbeck, T.; Houlihan, D. Quantitative analysis of alterations in liver ultrastructure of rainbow trout Oncorhynchus mykiss after administration of the aquaculture antibacterials oxolinic acid and flumequine. Dis. Aquat. Organ. 1997, 29, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Yin, G.J. Effects of high-fat diet on steatosis, endoplasmic reticulum stress and autophagy in liver of tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Zhong, J. Effects of Arachidonic Acid (ARA) on the Growth and Hepatoprotective of Monopterous albus; Jiangxi Agricultural University: Nanchang, China, 2018. [Google Scholar]
- Yin, G.J.; Cao, L.P.; Xu, P.; Jeney, G.; Nakao, M.; Lu, C.P. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2011, 37, 209–216. [Google Scholar] [CrossRef]
- Liu, X. Research and application of Chinese herbal medicine immunoenhancer. Mod. J. Ani. Husb. Vet. Med. 2004, 2, 31–32. [Google Scholar]
- Zhang, Q.R. Research progress in immune promotion of Chinese herbal medicine. J. Tradit. Chin. Vet. Med. 1997, 5, 15–16. [Google Scholar]
- Li, B.; Tang, Y.; Wang, Z.Q.; Zhang, Q. Control effect of Chinese herbal formula on grass carp hepatobiliary syndrome. South Chin. Fish. Sci. 2011, 7, 35–41. [Google Scholar]
- Ming, J.H.; Xie, J.; Xu, P.; Liu, W.B.; Zhou, Q.L.; Liu, B. Effects of emodin, vitamin C and their combination on biochemical parameters and two HSP70S mRNA expression of Wuchang bream (Megalobrama mblycephala) infected with Aeromonas hydrophila. J. Fish. Sci. Chin. 2011, 18, 588–601. [Google Scholar] [CrossRef]
- Wang, H.H. Research progress in prevention and treatment of aquatic animal diseases and pharmacology of Chinese herbal medicine. Chin. J. Vet. Med. 2004, 4, 37–41. [Google Scholar]
- Tian, L.L.; Wang, X.J.; Sun, Y.N.; Li, C.R.; Xing, Y.L.; Zhao, H.B.; Duan, M.; Zhe, Z.; Wang, S.Q. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int. J. Biochem. Cell Biol. 2008, 40, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Hong, C.Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011, 18, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xu, S.; Tian, X.Y. The Effect of Salvianolic Acid on Vascular Protection and Possible Mechanisms. Oxid. Med. Cell. Longev. 2020, 2020, 5472096. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Hu, Y.Y.; Liu, C.H.; Liu, P.; Zhu, D.Y. Effects of salvianolic acid B on expressions of TGF-beta1 and its receptors in liver of rats with dimethylnitrosamine-induced hepatic fibrosis. J. Chin. Integr. Med. 2005, 3, 286–289. [Google Scholar] [CrossRef]
- Xing, J.J.; Chen, X.; Tu, P.F.; Jiang, Y.; Zhao, J.Y. Effects of salvianolic acids on erythrocyte deformability in oleic acid induced acute lung injury in rabbits. Clin. Hemorheol. Microcirc. 2006, 34, 507–517. [Google Scholar]
- Yao, G.; Xu, L.; Wu, X.; Xu, L.; Yang, J.; Chen, H. Preventive effects of salvianolic acid B on transforming growth factor-beta1-induced epithelial-to-mesenchymal transition of human kidney cells. Biol. Pharm. Bull. 2009, 32, 882–886. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Du, G.H.; Zhang, J.T. Salvianolic acid B protects brain against injuries caused by schemia-reperfusion in rats. Acta Pharmacol. Sin. 2000, 21, 463–466. [Google Scholar]
- Liu, P.; Hu, Y.Y.; Liu, C.; Zhu, D.Y.; Xue, H.M.; Xu, Z.Q.; Xu, L.M.; Liu, C.H.; Gu, H.T.; Zhang, Z.Q. Clinical observation of salvianolic acid B in treatment of liver fibrosis in chronic hepatitis B. World J. Gastroenter. 2002, 8, 679–685. [Google Scholar] [CrossRef]
- Wang, Q.L.; Wu, Q.; Tao, Y.Y.; Liu, C.H.; EI-Nezami, H. Salvianolic acid B modulates the expression of drug-metabolizing enzymes in Hep G2 cells. Hepatob. Pancreat. Dis. Int. 2011, 10, 502–508. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.L.; Wang, L.; Cai, L.H.; Wu, H.F.; Wang, Y.W.; Gui, Z.X.; Li, L.; Tan, X.T. Sal B promotes apoptosis of human liver cancer cell line HepG2. Chin. J. Histochem. Cytochem. 2012, 21, 9–13. [Google Scholar]
- Gong, Y.; Wu, J.; Li, S.T. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immunosuppression in mice. Int. J. Clin. Exp. Med. 2015, 8, 20631–20637. [Google Scholar] [PubMed]
- Hua, X.M.; Zhou, H.Q.; Zhang, D.Q.; Li, N.L.; Yu, Q.W.; Shen, B.H. Immunoregulation of polysaccharide and probiotics on Fugu obscurus. J. Fish. Chin. 2006, 30, 230–235. [Google Scholar]
- Chen, Y.; Hua, X.M.; Zhang, D.Q.; Yu, Q.W.; Zhang, J.Y.; Bo, J.; Zhou, H.Q. Staudy on the immunomodulative function of chitosan and probiotics to the immunosuppressed gibel carp. Curr. Immunol. 2011, 31, 389–394. [Google Scholar]
- Deng, Z.T. Current situation of pharmacological and toxicological studies of cyclophosphamide. Tian. Phar. 2009, 21, 42–44. [Google Scholar]
- Bhanumathi, P.; Devi, P.U. Modulation of glutathione depletion and lipid peroxidation by WR-77913 an 2-mercaptopropionylglycine in cyclophosphamide chemotherapy. Indian J. Exp. Biol. 1994, 32, 562–564. [Google Scholar] [PubMed]
- Chen, Y.J.; Li, Y.; Xu, L.; Lin, Q.F.; Ge, X.X. Study on immunosuppression of cyclophosphamide on Paramisgurnus dabryanus. Chi. Feed 2015, 14, 26–29. [Google Scholar]
- Deleve, L.D. Cellular target of cyclophosphamide toxicity in the murine liver: Role of glutathione and site of metabolic activation. Hepatology 2010, 24, 830–837. [Google Scholar] [CrossRef]
- Ohno, Y.; Ormstad, K. Formation, toxicity and inactivation of acrolein during biotransformation of cyclophosphamide as studied in freshly isolated cells from rat liver and kidney. Arch. Toxicol. 1985, 57, 99–103. [Google Scholar] [CrossRef]
- Khan, S.; Ramwani, J.J.; O’Brien, P.J. Hepatocyte toxicity of mechlorethamine and other alkylating anticancer drugs: Role of lipid peroxidation. Biochem. Pharmacol. 1992, 43, 1963–1967. [Google Scholar] [CrossRef]
- Xu, B.; Wu, W.N.; Cui, X.L.; Wei, X.M.; Lai, Y.W.; Wang, Y.C.; Fan, H.Y. Protective effect of myricetin on cyclophosphamide induced liver injury in mice and its mechanism. Acta Nutr. Sin. 2020, 42, 178–182. [Google Scholar]
- Fu, J.; Zhao, D.; Lin, T.; Tian, Y.B. Effect of PAMK on apoptosis through mitochondrial pathway in chicken liver induced by CTX. J. Nor. Agric. Univ. 2019, 50, 63–70. [Google Scholar]
- Pan, H.; Yu, J.; Shi, Y.; Mao, Y.H.; Hu, S.H. Effect of ginseng stem-leaf saponins by oral administration on cyclophosphamide-induced oxidative stress in mice. J. Tradit. Chin. Vet. Med. 2015, 34, 45–47. [Google Scholar]
- Jin, J.Q.; Wu, S.Z.; Li, H.; Shen, M.Y. Effect of Salvia miltiorrhiza compound preparation on production performance and Newcastle disease antibody titer of broilers. Shan. J. Ani. Sci. Vet. Med. 2015, 36, 10–12. [Google Scholar]
- Lan, T.F. Studies on Tanshinone Components and Determination Method of Salvia miltiorrhiza; Shandong University of Traditional Chinese Medicine: Jinan, China, 2008. [Google Scholar]
- Xue, Q.; Liu, Y.; Li, Y.J.; Li, L.; Wang, S.H.; Xu, X.Y. The immunomodulatory and the protection of experimental liver injury of Lonicera macranthoides Hand: Mazz on mice. Chin. J. Pharm. 2013, 48, 601–605. [Google Scholar]
- Wu, Y.; Wang, F.; Zheng, Q.; Lu, L.; Yao, H.; Zhou, C.; Wu, X.; Zhao, Y. Hepatoprotective effect of total flavonoids from Laggera alata against carbon tetrachloride-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic damage. J. Biomed. Sci. 2006, 13, 569–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.H.; Ma, K.; Xu, X.B. A single dose of carbon monoxide intraperitoneal administration protects rat intestine from injury induced by lipopolysaccharide. Cell Stress Chaperones 2010, 15, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Qian, J.C.; Chen, S.Y.; Zhang, W.B.; Liu, C. Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress. Pharm. Biol. 2011, 49, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.P.; Du, J.L.; Jia, R.; Ding, W.D.; Xu, P.; Jeney, G.; Zhang, T. Effects of cyclophosphamide on antioxidative and immune functions of Nile tilapia (Oreochromis niloticus) via the TLR-NF-κB signaling pathway. Aquat. Toxicol. 2021, 239, 105956. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2013, 25, 402–408. [Google Scholar] [CrossRef]
- Puerto, M.; Gutiérrez-Praena, D.; Prieto, A.I.; Pichardo, S.; Jos, A.; Miguel-Carrasco, J.L.; Vazquez, C.M.; Cameán, A.M. Subchronic effects of cyanobacterial cells on the transcription of antioxidant enzyme genes in tilapia (Oreochromis niloticus). Ecotoxicology 2011, 20, 479–490. [Google Scholar] [CrossRef]
- Liang, Y.X.; Huang, L.D.; Huang, K.; Zhou, Y.; Cheng, Y.; Wu, L.H. Effects of fat, choline and feeding regime on catalase gene expression in the liver of GIFT (Oreochromis niloticus). Aquac. Res. 2018, 49, 1250–1261. [Google Scholar] [CrossRef]
- Habibi, E.; Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Keshavarz-Maleki, R.; Ahmadi, A. Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm. Biol. 2015, 53, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Malhotra, P.; Suri, V.; Varma, S.; Das, A.; Mitra, S. Cholestasis in a Patient of Multiple Myeloma: A Rare Occurrence of Bortezomib Induced Liver Injury. Indian J. Hematol. Blood Transfus. 2016, 32, 181–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Long, M.H.; Wu, J.; Wang, M.M.; Li, X.Y.; Shen, H.; Zhou, J.; Xu, L.; Fang, Z.J.; Luo, Y. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci. Rep. 2015, 5, 17536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Dong, C.R.; Zhang, Z.H.; Li, Q.; Zeng, H.H. Review of the research on 8-hydroxy-2 deoxyguanosine as a DNA oxidative damage marker. Chin. J. Clin. Pharm. 2017, 13, 1267–1270. [Google Scholar]
- Wang, Y.H.; Song, M.; Shi, L.I.; Hong, W. Effects of salvianolic acid B on liver fibrosis induced by carbon tetrachloride in rats. Chin. J. Mod. Med. 2014, 24, 24–28. [Google Scholar]
- Gao, H.Y.; Li, G.Y.; Lou, M.M.; Li, X.Y.; Wei, X.Y.; Wang, J.H. Hepatoprotective effect of Matrine salvianolic acid B salt on Carbon Tetrachloride-Induced Hepatic Fibrosis. J. Inflamm. 2012, 9, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Q.; Reng, Y.F.; Kong, W.Z.; Wang, Y.C. Effect of salvianolic acid B on oxidative stress in a cell model of nonalcoholic fatty liver disease. J. Clin. Hepatol. 2018, 34, 2175–2181. [Google Scholar]
- Li, W.G.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Zaman, M.B.; Leonard, M.O.; Ryan, E.J.; Nolan, N.P.; Hoti, E.; Maguire, D.; Mulcahy, H.; Traynor, O.; Taylor, C.T.; Hegarty, J.E.; et al. Lower expression of Nrf2 mRNA in older donor livers: A possible contributor to increased ischemia-reperfusion injury? Transplantation 2007, 84, 1272–1278. [Google Scholar] [CrossRef]
- Ren, Z.X.; Zhang, Y.Y. Research Progress on Salvianolic Acid B of the Chemical Composition and Pharmacological Effects. Sh. Chem. Ind. 2019, 48, 74–75. [Google Scholar]
- Zhang, P.C. Salvianolic Acid B Regulates Pyroptosis by the Nrf2 in Acute Kidney Injury. Master’s Thesis, Guangzhou University of Chinese Medicine, Guanzhou, China, 2019. [Google Scholar]
- Huang, Q.; Li, J.T.; Liu, Y.G.; Wei, H.L.; Yan, S.G.; Guo, Y.J.; Chang, Z.J. mTOR-related signalling pathway-Mediated autophagy in the regulation of liver injury. J. Clin. Hepatol. 2020, 36, 2621–2625. [Google Scholar]
- Wang, J.C. Studies on the Effects and Mechanisms of P38 Mediated Autophagy in Dasatinb Induced Hepatotoxicity; Zhejiang University: Hangzhou, China, 2014. [Google Scholar]
- Kundu, M.; Thompson, K. Autophagy: Basic Principles and elevance to Disease. Annu. Rev. Pathol. 2008, 3, 427–455. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of Acetaminophen-Induced Liver Necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar]
- Ke, P.Y. Diverse Functions of Autophagy in Liver Physiology and Liver Diseases. Int. J. Mol. Sci. 2019, 20, 300. [Google Scholar] [CrossRef] [Green Version]
- Rautou, P.; Cazals–Hatem, D.; Moreau, R.; Francoz, C.; Feldmann, G.; Lebrec, D.; Ogier–Denis, É.; Bedossa, P.; Valla, D.; Durand, F.O. Acute liver cell damage in patients with anorexia nervosa: A possible role of starvation-induced hepatocyte autophagy. Gastroenterology 2008, 135, 840–848. [Google Scholar] [CrossRef]
- Yang, M.C.; Chang, C.P.; Lei, H.Y. Endothelial cells are damaged by autophagic induction before hepatocytes in Con A-induced acute hepatitis. Int. Immunol. 2010, 22, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Amrein, L.; Soulières, D.; Johnston, J.B.; Aloyz, R. p53 and autophagy contribute to dasatinib resistance in primary CLL lymphocytes. Leuk. Res. 2011, 35, 99–102. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Cortés, J.; Kantarjian, H. Practical Management of Toxicities Associated with Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Clin. Lymphoma Myeloma 2008, 8, 82–88. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Lin, W.J.; Kuang, H.Y. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 2014, 10, 1692–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Wang, N.; Seto, S.W.; Chang, D.; Liang, H. Hydroxysafflor Yellow A Protects Brain Microvascular Endothelial Cells against Oxygen Glucose Deprivation/Reoxygenation Injury: Involvement of Inhibiting Autophagy via Class I PI3K/Akt/mTOR Signaling Pathway. Brain. Res. Bull. 2018, 140, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, Z.; Adriana, S.; Alberto, D.; Julia, R.; Shirley, T.; Michael, E.; Thomas, P.; Martin, S.; Riccardo, T.; Abul, K.T.; et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018, 37, e98308. [Google Scholar]
- He, T.T. Salvianolic Acid B Is Neuroprotecive through Mitigation of Autophagy and Apoptosis in Mouse Model of Focal Brain Ischemia; Hebei Medical University: Shijiazhuang, China, 2015. [Google Scholar]
- Hardie, D.G. AMPK-sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.W.; Yang, Y.Q.; Tang, L.; Wan, J.Y.; Dai, J.; Li, L.Q.; Huang, J.Y.; Shen, Y.; Lin, L.; Gong, X.Q.; et al. Therapeutic benefits of apocynin in mice with lipopolysaccharide/D-galactosamine-induced acute liver injury via suppression of the late stage pro-apoptotic AMPK/JNK pathway. Biomed. Pharmacother. 2020, 125, 110020. [Google Scholar] [CrossRef]
- Xie, T.F.; Wang, D.M.; Lu, Y. Research Progress of AMPK Signaling Pathway in Alcoholic Fatty Liver. Chin. J. Cell Biol. 2020, 42, 1221–1228. [Google Scholar]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.G.; Hurley, J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol. 2016, 39, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Chao, X.; Wang, S.; Zhao, K.; Yuan, L.; Williams, J.A.; Li, T.; Hemantkumar, C.; Partha, K.; He, X.C.; Li, L. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 2018, 155, 865–879. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Wei, C. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef] [Green Version]
- Xin, M.Y. Protection of Autophagy in Ischemic Brain Injury and the Neuroprotective Mechanism of Salvianolic Acid B Base on AMPK/m TOR/ULK1 Pathway; Jilin University: Changchun, China, 2020. [Google Scholar]
- Tang, X.; Dai, Y.; Yang, X.Z. Effects of salvianolic acid B on high glucose-induced apoptosis and autophagy of retinal Müller cells and AMPK signaling pathway. Recent Adv. Ophthalmol. 2022, 42, 122–127. [Google Scholar]
- An, L.P.; Yu, K.; Gen, H.B.; Wang, W. Advances in research on mechanism of salvianolic acid B against oxidative stress. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 227–234. [Google Scholar]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Hamivand, Z.; Haddadi, G.; Fardid, R. Expression of Bax and Bcl2 Genes in Peripheral Blood Lymphocytes of Patients with Differentiated Thyroid Cancer. J. Med. Phys. 2018, 43, 41–45. [Google Scholar] [PubMed]
- Yang, T.; Fei, Z.H.; Zhong, X.M. Research progress of caspase family and apoptosis. Zj. Med. J. 2018, 40, 96–100. [Google Scholar]
- Yao, L.L. Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Selenium Deficiency-Induced Apoptosis in Chicken Liver; Northeast Agricultural University: Harbin, China, 2015. [Google Scholar]
- Zhang, F.Y. The Protective Effects and Underlying Mechanism of Sal B against Hydrogen Eroxide Induced Apoptosis in HUVECs; Southwest Medical University: Luzhou, China, 2018. [Google Scholar]
- Yan, X.C.; Chen, Q.; Shen, L.; Tao, Y.Y.; Liu, C.H. Effect of salvianolic acid B on hepatocyte apoptosis and its mechanism. In Proceedings of the National Academic Conference on Integrated Chinese and Western Medicine for Liver Diseases, Shanghai, China, September 2008. [Google Scholar]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2017, 80, 50–64. [Google Scholar]
- An, J.J.; Shi, K.J.; Wei, W.; Hua, F.Y.; Ci, Y.L.; Jiang, Q.; Li, F.; Wu, P.; Hui, K.Y.; Yang, Y. The ROS/jnk/ATF2 pathway mediates selenite-induced leukemia NB4 cell cycle arrest and apoptosis in vitro and in vivo. Cell Death Dis. 2017, 4, e973. [Google Scholar] [CrossRef] [Green Version]
- Stander, X.X.; Strander, B.A.; Joubert, A.M. Synergistic anticancer potential of dichloroacetate and estradiol analogue exerting their effect via ROS-JNK-Bcl-2-mediated signalling pathways. Cell Physiol. Biochem. 2015, 35, 1499–1526. [Google Scholar] [CrossRef]
- Zheng, S.G.; Zhu, Y.M.; Tao, S.J.; Zheng, H.W.; Reng, Y.N.; Zhao, M.Q.; Yang, J.R.; Wu, Y.J. Effects of salvianolic acid B on JNK activation and INS-1 cell apoptosis induced by intermittent high glucose. Chin. Pharmacol. Bull. 2017, 33, 68–73. [Google Scholar]







| Component/g·kg−1 | Control | 0.25 g·kg−1 Sal B | 0.50 g·kg−1 Sal B | 0.75 g·kg−1 Sal B |
|---|---|---|---|---|
| Fish meal | 60.0 | 60.0 | 60.0 | 60.0 |
| Rapeseed meal | 270.0 | 270.0 | 270.0 | 270.0 |
| Flour | 174 | 173.75 | 173.50 | 173.25 |
| Sal B | 0 | 0.25 | 0.50 | 0.75 |
| Rice bran meal | 100.0 | 100.0 | 100.0 | 100.0 |
| Cottonseed meal | 100.0 | 100.0 | 100.0 | 100.0 |
| Soyabean meal | 240 | 240 | 240 | 240 |
| Phospholipid | 10 | 10 | 10 | 10 |
| Soybean oil | 10 | 10 | 10 | 10 |
| Monocalcium phosphate | 15 | 15 | 15 | 15 |
| Choline chloride | 1 | 1 | 1 | 1 |
| Vitamin premix 1 | 10 | 10 | 10 | 10 |
| Mineral premix 2 | 10 | 10 | 10 | 10 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Approximate composion | ||||
| Crude protein/% | 31.6 | 31.6 | 31.6 | 31.6 |
| Crude lipid/% | 4.6 | 4.6 | 4.6 | 4.6 |
| Ash/% | 6.4 | 6.4 | 6.4 | 6.4 |
| Gene | Primer Sequence (5′-3′) | GenBank Number/References |
|---|---|---|
| nrf2 | F: CTGCCGTAAACGCAAGATGG | XM_003447296.4 |
| R: ATCCGTTGACTGCTGAAGGG | ||
| keap1 | F: CTTCGCCATCATGAACGAGC | XM_003447926.3 |
| R: CACCAACTCCATACCGCACT | ||
| ucp2 | F: CAGGGATCGTTACTCGGCTC | XM_019363847.2 |
| R: CCGTTGTATCTCCTCTCGCC | ||
| ho-1 | F: CTTGCCCGTGTGGAATCACT | XM_013270165.2 |
| R: AGATCACCGAGGTAGCGAGT | ||
| gpx3 | F: CGATGTGGCCCGTGTTACC | XM_005467951.3 |
| R: CAGCCTTGCCTGCGTAGT | ||
| gclc | F: ATCGAGGGAACTCCAGGTCA | XM_003441123.5 |
| R: GGAGTTGGTCGGTACTCTGG | ||
| gsr | F: TACGCTGTCGGGGATGTTTG | XM_003445184.5 |
| R: AATGGCCTCGTCCTCTGTGA | ||
| gstα | F: TAATGGGAGAGGGAAGATGG | NM_001279635.1 [42] |
| R: CTCTGCGATGTAATTCAGGA | ||
| cat | F: ATGGCCACCGTCACATGAAT | XM_019361816.2 |
| R: CAAACGGGTTGAACCGGAAC | ||
| jnk1 | F: CAAGCCCGTGACCTCCTATC | XM_025909605.1 |
| R: TCCATTCCTCCACTGTGTGC | ||
| jnk2 | F: GCACGGGATTTGCTTTCCAA | XM_005467784.4 |
| R: TGTGCTCTCTCTCTTCCAGC | ||
| ask1 | F: TCTCCCACGAATCCCACAATG | XM_003446319.5 |
| R: GCTCAGTTCGCAGCTTCAGT | ||
| p38 | F: GGATGACCACGTCCAGTTCC | XM_003441528.5 |
| R: TTTCATCATCCGTGTGCCGT | ||
| erk2 | F: CGAAGGGCTACACCAAGTCC | XM_003444474.5 |
| R: GATGATGCAGTTGAGATCCTCCT | ||
| cas3 | F: CAGACAGCGAAACTGATGGTG | NM_001282894.1 |
| R: CACCTTGTGGTTCACTCGGG | ||
| cas8 | F: AGGGTGTAGTTTTGGGAGCTG | XM_019348156.2 |
| R: GGGGATCGTCAATGGTGAAGTA | ||
| cas9 | F: AGACGGACGCTATTCCGATG | XM_025901776.1 |
| R: CGCCAAGAAACATAACCTGGG | ||
| cytc | F: GACGCCAACAAGAGCAAAGG | XM_005473697.4 |
| R: AATGAGGTCTTGGCGCTCTC | ||
| Bcl2 | F: AGACTGTACCAGCCGGACTT | XM_003437902.5 |
| R: GTGCCCCCAAACTCGAAGAA | ||
| bax | F: TGGCAATAAAGCAGTGACGAG | XM_019357746.2 |
| R: AGGCCACTCTCATAAAAACCTC | ||
| p53 | F: GGCAATCAGAGGGCTCAGTA | XM_025905405.1 |
| R: GTGAGGATAGGTCTGCGGTT | ||
| ampkα | F: GTGGGGTGATTCTCTACGCC | NM_001319868.1 |
| R: GGCTCTTTTCATCGGGTCCA | ||
| mtor | F: AGCGACAGTGAGGTTGACAG | XM_003449131.5 |
| R: GGACAGTGAAATGGAGCGGA | ||
| ulk1 | F: GGTCCAGAATTACCAGCGCA | XM_019361142.2 |
| R: CCCATAGGGTGGTGAAGGTC | ||
| mcoln-1 | F: CTGCTGTGTGGCTGTCATCT | XM_005470179.4 |
| R: GACTGGTCTCCTGCATCTCTG | ||
| lamp1 | F: CTGGTTTTGACACAGACGCAA | XM_003445782.5 |
| R: ATGTAGGAGTAGCCCAGCGT | ||
| uvrag | F: ATCACCCACACACCTGTCATC | XM_003458979.5 |
| R: TGTTCAGCGGTTTTACGTCCT | ||
| tfeb | F: GCACCTACCCCATAACCAGG | XM_019358786.2 |
| R: GCAAAGTCAAAGTGCTGGGAG | ||
| atg3 | F: CGACTCTGGCTCTTTGGATATGA | XM_003454721.5 |
| R: CCTTCCGCCACAGTCTCAAT | ||
| atg5 | F: GGATGGGCTTGCAGAACGATA | XM_003450274.5 |
| R: AAGGGTGTATGCGTTGCCT | ||
| atg7 | F: TCTCTCAGACCACTCTGTCCC | XM_025907253.1 |
| R: AGCAGCATTCACCACTAGCTT | ||
| atg13 | F: CGATGATGATGGCTTGTCGC | XM_005460641.4 |
| R: AAACGCAGCAAATGGCAGG | ||
| lc3b | F: GCACCCCAACAAAATACCTGT | XM_003439438.5 |
| R: CCTGGTTGGAGTTTAGCTGGA | ||
| beclin1 | F: ACCATCAACAACTTCCGCCT | XM_005471281.3 |
| R: TCTGAAAGTGCAGCCCCATT | ||
| p62 | F: CCCTTCTAAACCTGCTGCTGA | XM_005463795.4 |
| R: TCACCTTGGTCCGTTGGC | ||
| β-actin | F: CCTGAGCGTAAATACTCCGTCTG | KJ126772.1 [43] |
| R: AAGCACTTGCGGTGGACGAT |
| Group | Initial Body Weight/g | Final Weight/g | RWR/% | SGR/(%·d−1) | FCR |
|---|---|---|---|---|---|
| Control | 30.04 ± 1.09 | 89.12 ± 3.03 a | 196.67 ± 5.71 a | 1.81 ± 0.05 a | 2.96 ± 0.17 a |
| CTX | 30. 08 ± 1.13 | 92.88 ± 2.69 a | 208.78 ± 6.05 a | 1.88 ± 0.07 a | 2.78 ± 0.11 a |
| 0.25 g·kg−1 Sal B | 30.00 ± 0.92 | 97.80 ± 3.21 ab | 226.00 ± 6.39 ab | 1.97 ± 0.12 b | 2.58 ± 0.12 ab |
| 0.50 g·kg−1 Sal B | 29.75 ± 1.05 | 98.14 ± 3.08 b | 229.88 ± 6.39 b | 1.99 ± 0.08 b | 2.55 ± 0.10 b |
| 0.75 g·kg−1 Sal B | 29.91 ± 0.89 | 100.24 ± 3.57 b | 235.14 ± 8.01 b | 2.02 ± 0.13 b | 2.48 ± 0.13 b |
| Tissues | Parameters | Control | CTX | 0.25 g·kg−1 Sal B | 0.50 g·kg−1 Sal B | 0.75 g·kg−1 Sal B |
|---|---|---|---|---|---|---|
| Serum | GPT/IU·L−1 | 3.24 ± 0.48 b | 8.12 ± 1.64 a | 6.11 ± 0.82 a | 4.77 ± 0.63 ab | 3.17 ± 0.36 b |
| GOT/IU·L−1 | 3.15 ± 0.22 c | 9.78 ± 0.77 a | 7.46 ± 0.80 b | 4.17 ± 0.53 c | 4.00 ± 0.56 c | |
| TP/g·L−1 | 22.12 ± 0.94 a | 15.77 ± 0.63 c | 17.97 ± 1.12 bc | 18.54 ± 1.24 bc | 21.17 ± 1.73 ab | |
| Alb/g·L−1 | 16.08 ± 0.89 a | 12.31 ± 0.74 c | 12.97 ± 0.44 bc | 14.66 ± 0.94 ab | 15.05 ± 0.88 ab | |
| T-AOC/mmol·L−1 | 2.18 ± 0.18 a | 0.76 ± 0.02 c | 0.76 ± 0.05 c | 1.08 ± 0.13 c | 1.54 ± 0.15 b | |
| Liver | 8-OHdG/ng·g prot−1 | 274.13 ± 9.12 c | 332.41 ± 12.10 a | 328.98 ± 10.34 ab | 303.49 ± 15.64 abc | 295.99 ± 14.24 bc |
| NO/μM·mg prot−1 | 5.92 ± 0.36 a | 3.54 ± 0.57 b | 3.81 ± 0.33 b | 4.74 ± 0.58 ab | 5.08 ± 0.46 a | |
| T-AOC/μmol·g prot−1 | 79.91 ± 5.70 a | 49.52 ± 3.86 c | 65.04 ± 3.50 b | 72.02 ± 6.42 ab | 75.24 ± 5.34 ab | |
| SOD/U·mg prot−1 | 92.54 ± 8.39 a | 69.00 ± 3.05 c | 70.90 ± 6.95 bc | 87.83 ± 8.48 ab | 88.11 ± 4.03 ab | |
| CAT/U·mg prot−1 | 800.58 ± 17.67 a | 641.55 ± 24.21 c | 644.84 ± 41.62 c | 668.84 ± 49.38 bc | 770.36 ± 57.97 ab | |
| MDA/nmol·mg port−1 | 0.37 ± 0.02 b | 0.62 ± 0.03 a | 0.58 ± 0.07 a | 0.52 ± 0.03 a | 0.40 ± 0.04 b | |
| GSH/μmol ·g prot−1 | 40.06 ± 9.88 a | 13.94 ± 1.76 c | 15.42 ± 8.16 bc | 26.81 ± 9.76 abc | 38.98 ± 9.74 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Yin, G.; Du, J.; Jia, R.; Gao, J.; Shao, N.; Li, Q.; Zhu, H.; Zheng, Y.; Nie, Z.; et al. Salvianolic Acid B Regulates Oxidative Stress, Autophagy and Apoptosis against Cyclophosphamide-Induced Hepatic Injury in Nile Tilapia (Oreochromis niloticus). Animals 2023, 13, 341. https://doi.org/10.3390/ani13030341
Cao L, Yin G, Du J, Jia R, Gao J, Shao N, Li Q, Zhu H, Zheng Y, Nie Z, et al. Salvianolic Acid B Regulates Oxidative Stress, Autophagy and Apoptosis against Cyclophosphamide-Induced Hepatic Injury in Nile Tilapia (Oreochromis niloticus). Animals. 2023; 13(3):341. https://doi.org/10.3390/ani13030341
Chicago/Turabian StyleCao, Liping, Guojun Yin, Jinliang Du, Rui Jia, Jiancao Gao, Nailin Shao, Quanjie Li, Haojun Zhu, Yao Zheng, Zhijuan Nie, and et al. 2023. "Salvianolic Acid B Regulates Oxidative Stress, Autophagy and Apoptosis against Cyclophosphamide-Induced Hepatic Injury in Nile Tilapia (Oreochromis niloticus)" Animals 13, no. 3: 341. https://doi.org/10.3390/ani13030341