Refining Stereotaxic Neurosurgery Techniques and Welfare Assessment for Long-Term Intracerebroventricular Device Implantation in Rodents
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Implantable Devices
2.3. Intrathecal Implantable Device Surgery
2.3.1. Preoperative Care and Treatment
2.3.2. Stereotaxic Surgery Procedure
2.3.3. Postoperative Care
2.4. Body Weight Monitoring and Welfare Assessment
2.5. Behavioral Analysis
2.6. Catheter Placement Validation
2.6.1. In Vivo Dye Infusion and Visualization
2.6.2. Histological Analysis
2.7. Statistics
3. Results
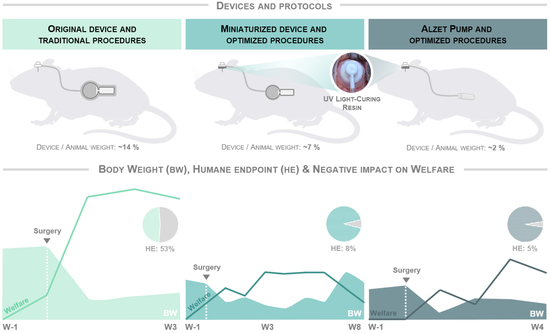
3.1. The Optimized Protocol of Stereotaxic Surgeries Shortens the Recovery Time Required after Surgery and Significantly Minimizes Humane Endpoint Application
3.2. Improved Protocol Reduces the Drop in Body Weight Observed in Mice from the First Postoperative Surgery Onwards and Normalizes It over Time
3.3. Optimized Protocol and Implantation of Smaller Devices Significantly Improve Animal Welfare and Enable Longer and Safer Experimental Periods
3.4. Optimized Intrathecal Implantation Procedures Do Not Appear to Negatively Affect General and Anxiety-like Behaviors for at Least Two Months after Surgery
3.5. Validation of Cannula Placement Is Required after Experiment Termination to Confirm Correct Implantation at the Stereotaxic Coordinates of Interest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferry, B.; Gervasoni, D. Improving Stereotaxic Neurosurgery Techniques and Procedures Greatly Reduces the Number of Rats Used per Experimental Group—A Practice Report. Animals 2021, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Coto-Vilcapoma, M.A.; Castilla-Silgado, J.; Fernández-García, B.; Pinto-Hernández, P.; Cipriani, R.; Capetillo-Zarate, E.; Menéndez-González, M.; Álvarez-Vega, M.; Tomás-Zapico, C. New, Fully Implantable Device for Selective Clearance of CSF-Target Molecules: Proof of Concept in a Murine Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 9256. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Kuo, H.Y.; Liu, F.C. Stereotaxic Surgery for Genetic Manipulation in Striatal Cells of Neonatal Mouse Brains. J. Vis. Exp. 2018, 137, e57270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVos, S.L.; Miller, T.M. Direct Intraventricular Delivery of Drugs to the Rodent Central Nervous System. J. Vis. Exp. 2013, 75, e50326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, B.M.; Frank, L.E.; Caldera-Siu, A.D.; Pothos, E.N. Survivable Stereotaxic Surgery in Rodents. J. Vis. Exp. 2008, 20, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, K.; Madsen, P.J.; Smith, T.; Griffin, C.; Patterson, L.; Vitanza, N.A.; Storm, P.B.; Resnick, A.C.; Foster, J.B. Intracranial Cannula Implantation for Serial Locoregional Chimeric Antigen Receptor (CAR) T Cell Infusions in Mice. J. Vis. Exp. 2023, 192, e64886. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.I.; McGavin, J.J.; Cochkanoff, N.L.; Crosby, K.M. Stereotaxic Surgery for Implantation of Guide Cannulas for Microinjection into the Dorsomedial Hypothalamus in Young Rats. MethodsX 2019, 6, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Fornari, R.V.; Wichmann, R.; Atsak, P.; Atucha, E.; Barsegyan, A.; Beldjoud, H.; Messanvi, F.; Thuring, C.M.A.; Roozendaal, B. Rodent Stereotaxic Surgery and Animal Welfare Outcome Improvements for Behavioral Neuroscience. J. Vis. Exp. 2012, 59, e3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado, A.; Barford, J.; Parker, F.; Bate, S.; Whelan, G. Use of Cyanoacrylate Gel as a Substitute for Dental Cement in Intracerebroventricular Cannulations in Rats. Contemp. Top. Lab. Anim. Sci. 2003, 42, 13–16. [Google Scholar]
- Sike, Á.; Wengenroth, J.; Upīte, J.; Brüning, T.; Eiriz, I.; Sántha, P.; Biverstål, H.; Jansone, B.; Haugen, H.J.; Krohn, M.; et al. Improved Method for Cannula Fixation for Long-Term Intracerebral Brain Infusion. J. Neurosci. Methods 2017, 290, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical Applications of Methyl Methacrylate: A Review of Toxicity. Arch. Environ. Occup. Health 2009, 64, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Díez-Solinska, A.; Vegas, O.; Azkona, G. Refinement in the European Union: A Systematic Review. Animals 2022, 12, 3263. [Google Scholar] [CrossRef]
- Russel, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co., Ltd.: London, UK, 1959. [Google Scholar]
- Arman, A.; Hutchinson, M.R. Intrathecal Implantation Surgical Considerations in Rodents; a review. J. Neurosci. Methods 2021, 363, 109327. [Google Scholar] [CrossRef] [PubMed]
- Aulehner, K.; Leenaars, C.; Buchecker, V.; Stirling, H.; Schönhoff, K.; King, H.; Häger, C.; Koska, I.; Jirkof, P.; Bleich, A.; et al. Grimace Scale, Burrowing, and Nest Building for the Assessment of Post-Surgical Pain in Mice and Rats—A Systematic Review. Front. Vet. Sci. 2022, 9, 930005. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Torres-Lista, V. Social Nesting, Animal Welfare, and Disease Monitoring. Animals 2021, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Morton, D.B.; Burman, O.; Dennison, N.; Honess, P.; Jennings, M.; Lane, S.; Middleton, V.; Roughan, J.V.; Wells, S.; et al. A Guide to Defining and Implementing Protocols for the Welfare Assessment of Laboratory Animals: Eleventh Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 2011, 45, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Meer, M.; Rolls, A.; Baumans, V.; Olivier, B.; van Zutphen, L.F.M. Use of Score Sheets for Welfare Assessment of Transgenic Mice. Lab. Anim. 2001, 35, 379–389. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; Pitzer, C.; Lewejohann, L.; Jirkof, P.; Siegeler, K.; Segelcke, A.; Drude, N.; Pradier, B.; Pogatzki-Zahn, E.; Hollinderbäumer, B.; et al. The “WWHow” Concept for Prospective Categorization of Post-Operative Severity Assessment in Mice and Rats. Front. Vet. Sci. 2022, 9, 841431. [Google Scholar] [CrossRef]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef] [Green Version]
- Jirkof, P.; Rudeck, J.; Lewejohann, L. Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research? Animals 2019, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- FELASA Working Group; Mähler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA Recommendations for the Health Monitoring of Mouse, Rat, Hamster, Guinea Pig and Rabbit Colonies in Breeding and Experimental Units. Lab. Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32658/ (accessed on 27 June 2023). [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant Presenilins Specifically Elevate the Levels of the 42 Residue β-Amyloid Peptide in Vivo: Evidence for Augmentation of a 42-Specific γ Secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Menéndez-González, M.; Popescu, B.O. The “Cerebrospinal Fluid Sink Therapeutic Strategy” in Alzheimer’s Disease—From Theory to Design of Applied Systems. Biomedicines 2022, 10, 1509. [Google Scholar] [CrossRef]
- Menéndez-González, M.; Tamba, B.-I.; Leclere, M.; Mabrouk, M.; Schreiner, T.-G.; Ciobanu, R.; Cristina, T.-Z. Intrathecal Pseudodelivery of Drugs in the Therapy of Neurodegenerative Diseases: Rationale, Basis and Potential Applications. Pharmaceutics 2023, 15, 768. [Google Scholar] [CrossRef]
- Menéndez-González, M. Mechanical Filtration of the Cerebrospinal Fluid: Procedures, Systems, and Applications. Expert Rev. Med. Devices 2023, 20, 199–207. [Google Scholar] [CrossRef]
- ISO Standard. Available online: https://www.iso.org/iso-13485-medical-devices.html (accessed on 6 August 2023).
- ISO Standard. Available online: https://www.iso.org/standard/68936.html (accessed on 6 August 2023).
- Gurfein, B.T.; Stamm, A.W.; Bacchetti, P.; Dallman, M.F.; Nadkarni, N.A.; Milush, J.M.; Touma, C.; Palme, R.; di Borgo, C.P.; Fromentin, G.; et al. The Calm Mouse: An Animal Model of Stress Reduction. Mol. Med. 2012, 18, 606–617. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, K.S.; Jo, W. Stress Evaluation of Mouse Husbandry Environments for Improving Laboratory Animal Welfare. Animals 2023, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Rufiange, M.; Leung, V.S.; Simpson, K.; Pang, D.S. Prewarming Followed by Active Warming Is Superior to Passive Warming in Preventing Hypothermia for Short Procedures in Adult Rats (Rattus Norvegicus) under Isoflurane Anesthesia. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 377–383. [Google Scholar] [CrossRef]
- Bailey, K.T.; Jantre, S.R.; Lawrence, F.R.; Hankenson, F.C.; Del Valle, J.M. Evaluation of Active Warming and Surgical Draping for Perioperative Thermal Support in Laboratory Mice. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 482–494. [Google Scholar] [CrossRef]
- Schuster, C.J.; Pang, D.S.J. Forced-Air Pre-Warming Prevents Peri-Anaesthetic Hypothermia and Shortens Recovery in Adult Rats. Lab. Anim. 2018, 52, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Gervasoni, D.; Vogt, C. Stereotaxic Neurosurgery in Laboratory Rodent; Springer: Paris, France, 2014; ISBN 978-2-8178-0471-2. Available online: https://link.springer.com/book/10.1007/978-2-8178-0472-9 (accessed on 27 June 2023).
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef] [PubMed]
- Ullman-Culleré, M.H.; Foltz, C.J. Body Condition Scoring: A Rapid and Accurate Method for Assessing Health Status in Mice. Lab. Anim. Sci. 1999, 49, 319–323. [Google Scholar] [PubMed]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining Body-Weight Reduction as a Humane Endpoint: A Critical Appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; McCabe, J.T. Behavior of Male and Female C57Bl/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated plus Maze. Front. Behav. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šakić, B. Cerebrospinal Fluid Collection in Laboratory Mice: Literature Review and Modified Cisternal Puncture Method. J. Neurosci. Methods 2019, 311, 402–407. [Google Scholar] [CrossRef]
- Talbot, S.R.; Struve, B.; Wassermann, L.; Heider, M.; Weegh, N.; Knape, T.; Hofmann, M.C.J.; von Knethen, A.; Jirkof, P.; Häger, C.; et al. RELSA—A Multidimensional Procedure for the Comparative Assessment of Well-Being and the Quantitative Determination of Severity in Experimental Procedures. Front. Vet. Sci. 2022, 9, 937711. [Google Scholar] [CrossRef]










| Parameter and Time Analyzed | Naïve | Original Device | Miniaturized Device | Alzet Pump a | ||||
|---|---|---|---|---|---|---|---|---|
| WT | APP | WT | APP | WT | APP | WT | APP | |
| Recovery time after surgery | 10 | 10 | 9 | 10 | 3 | 10 | 10 | 10 |
| Humane endpoint application | 10 | 10 | 9 | 10 | 3 | 10 | 10 | 10 |
| Body weight at W1 | 10 | 10 | 9 | 10 | 3 | 10 | 10 | 10 |
| Body weight at W3 | 10 | 10 | 7 | 7 | 3 | 9 | 10 | 9 |
| Body weight at W8 | 5 | 5 | - | - | 3 | 9 | - | - |
| % Change in body weight at W0 | 10 | 10 | 9 | 10 | 3 | 10 | 10 | 10 |
| % Change in body weight at W3 | 10 | 10 | 7 | 7 | 3 | 9 | 10 | 9 |
| % Change in body weight at W8 | 5 | 5 | - | - | 3 | 9 | - | - |
| Welfare assessment score at W3 | 10 | 10 | 7 | 7 | 3 | 9 | 10 | 9 |
| Welfare assessment score at W8 | 5 | 5 | - | - | 3 | 9 | - | - |
| Behavioral testing at W1 | 10 | 10 | - | - | 3 | 9 | 9 | 10 |
| Behavioral testing at W4/5 | 10 | 10 | - | - | 3 | 9 | 9 | 9 |
| Behavioral testing at W8 | 6 | 5 | - | - | 3 | 9 | - | - |
| Parameter | Original Device | Miniaturized Device | ALZET® Pump 1004 a |
|---|---|---|---|
| Weight (filled; g) | 4.29 ± 0.41 | 2.25 ± 0.15 | 0.56 ± 0.06 |
| % Weight (with respect to animal body weight b; %) | 14.16 ± 1.35 | 6.93 ± 0.46 | 1.85 ± 0.19 |
| Total Volume (cm3) | 5.92 | 2.54 | 0.54 |
| Maximum L (cm) | 3.5 | 2.9 | 1.5 |
| Maximum W (cm) | 1.5 | 1.3 | 0.6 |
| Maximum H (cm) | 1.5 | 0.9 | 0.6 |
| Coated Material | Polyetheretherketone (PEEK; ISO 13485 [29]) | LOCTITE® SI 5248 silicone (Henkel, Düsseldorf, Germany; ISO 10993 [30]) | Cellulose ester blend |
| Catheter Tube Length and Outside Diameter (cm) | 3 ± 0.2, 0.11 ± 0.008 | 3 ± 0.2, 0.11 ± 0.008 | 3 ± 0.2, 0.11 ± 0.008 |
| Catheter Tube Material and Configuration | Polyvinyl chloride (MG), pre-attached | Polyvinyl chloride (MG), pre-attached | Polyvinyl chloride (MG), attachable |
| Brain Infusion III Cap Cannula Dimensions (L, W, H; cm) c | 0.59, 0.59, 0.2 | 0.59, 0.59, 0.2 | 0.59, 0.59, 0.2 |
| Brain Infusion III Cap Material c | Polycarbonate | Polycarbonate | Polycarbonate |
| Brain Infusion III Cannula Dimensions (L, W, H; cm) c | 0.3, 0.031, 0.031 | 0.3, 0.031, 0.031 | 0.3, 0.031, 0.031 |
| Brain Infusion III Cannula Material c | Stainless steel | Stainless steel | Stainless steel |
| Parameter | Original Device | Miniaturized Device | ALZET® Pump 1004 a |
|---|---|---|---|
| Apheresis Module Dimensions (L, W, H; cm) | 1.4, 1.5, 1.5 | 1.3, 1.3, 0.9 | - |
| Apheresis Module External Volume (cm3) | 3.150 | 1.521 | - |
| Apheresis Module Internal Volume (µL) | 235.5 | 235.5 | - |
| Reservoir Dimensions (L, W, H; cm) | 2.1, 1.2, 1.1 | 1.6, 0.8, 0.8 | 1.5, 0.6, 0.6 |
| Reservoir External Volume (cm3) | 2.772 | 1.024 | 0.540 |
| Reservoir Internal Volume (µL) | 100 | 100 | 100 |
| Total Volume (cm3) | 5.922 | 2.545 | 0.540 |
| Parameters | Score | |
|---|---|---|
| General Condition, Physical Appearance, and Posture | ||
| Smooth and shiny fur, clean forelimbs, and nose | 0 | |
| Presence of piloerection, unkempt fur | 1 | |
| Abnormal posture (abdominal curvature and/or kyphosis, increased muscle tone) | 2 | |
| Skin lesions unrelated to surgery (ear dermatitis, scratches, excessive barbering) | 2 | |
| Nutritional and Hydration Status | ||
| Unaffected or increased | 0 | |
| Dehydration signs | <2 s after pinching the back skin | 1 |
| >2 s after pinching the back skin | 2 | |
| Animal Body weight | 1 ≤ 10% weight loss | 1 |
| 10–20% weight loss | 4 | |
| >20% weight loss (duration >4 postoperative days) | 8 | |
| Spontaneous Behavior | ||
| Normal behavior (sleeping, exploration, grooming, nesting, interaction with environmental enrichment objects) | 0 | |
| No use of enrichment objects, no nesting behavior | 1 | |
| Impairment of motor function (hypo-locomotion) | 4 | |
| Lethargy and/or slight loss of balance | 8 | |
| Hind-limbs paralysis, tremors and/or signs of vestibulocochlear dysfunction | 12 | |
| Surgery-specific Parameters | ||
| Clean and dry surgical incision, no signs of infection, no pain or signs of distress | 0 | |
| Scratches around the scar or slight redness | 1 | |
| Redness and/or necrosis of the skin around the scar | 3 | |
| Grimace scale | Moderate | 4 |
| Severe | 6 | |
| Suture opening Grade I (loose stitches with closed and healed wound) | 3 | |
| Suture opening Grade II (loose stitches with unhealed wound) | 6 | |
| Suture opening Grade III (open unhealed wound) | 10 | |
| Suture opening Grade IV (Brain Infusion Kit cannula disconnected) | 12 | |
| Parameter | Comparisons and p-Values | |||
|---|---|---|---|---|
| Recovery time | Original Device | Miniaturized Device | Alzet Pump | |
| >20 min | <0.001 | 0.002 | <0.001 | |
| 5–10 min | 0.284 | 0.003 | 0.151 | |
| <5 min | <0.001 | 0.341 | <0.001 | |
| Naïve | Original Device | Miniaturized Device | Alzet Pump | |
| Humane endpoint | 0.029 | <0.001 | 0.681 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Martín, E.; Coto-Vilcapoma, A.; Castilla-Silgado, J.; Rodríguez-Cañón, M.; Prado, C.; Álvarez, G.; Álvarez-Vega, M.A.; Fernández-García, B.; Menéndez-González, M.; Tomás-Zapico, C. Refining Stereotaxic Neurosurgery Techniques and Welfare Assessment for Long-Term Intracerebroventricular Device Implantation in Rodents. Animals 2023, 13, 2627. https://doi.org/10.3390/ani13162627
Pérez-Martín E, Coto-Vilcapoma A, Castilla-Silgado J, Rodríguez-Cañón M, Prado C, Álvarez G, Álvarez-Vega MA, Fernández-García B, Menéndez-González M, Tomás-Zapico C. Refining Stereotaxic Neurosurgery Techniques and Welfare Assessment for Long-Term Intracerebroventricular Device Implantation in Rodents. Animals. 2023; 13(16):2627. https://doi.org/10.3390/ani13162627
Chicago/Turabian StylePérez-Martín, Ester, Almudena Coto-Vilcapoma, Juan Castilla-Silgado, María Rodríguez-Cañón, Catuxa Prado, Gabriel Álvarez, Marco Antonio Álvarez-Vega, Benjamín Fernández-García, Manuel Menéndez-González, and Cristina Tomás-Zapico. 2023. "Refining Stereotaxic Neurosurgery Techniques and Welfare Assessment for Long-Term Intracerebroventricular Device Implantation in Rodents" Animals 13, no. 16: 2627. https://doi.org/10.3390/ani13162627