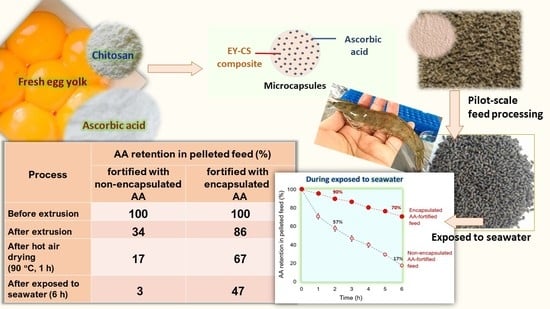
Improvement of Moist Heat Resistance of Ascorbic Acid through Encapsulation in Egg Yolk–Chitosan Composite: Application for Production of Highly Nutritious Shrimp Feed Pellets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microencapsulation of AA in EY-CS Composites
2.3. Determination of Production Yield
2.4. Determination of Encapsulation Efficiency
2.5. Determination of Moist Heat Resistance of Microcapsules
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Determination of Microcapsules Morphology
2.8. Determination of Particle Size Distribution
2.9. Production of Pelleted Feed and Evaluation of Its Characteristics
2.9.1. Production of Pelleted Feeds
2.9.2. Determination of Moisture Content and Bulk Density
2.9.3. Determination of Sinking Velocity
2.9.4. Determination of AA Content
2.9.5. Determination of Stability of Pelleted Feeds and Leaching of AA into Seawater
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of the Ratio of EY to AA on Production Yield, Encapsulation Efficiency, and Moist Heat Resistance of Microcapsules
3.2. Effects of CS as a Coencapsulation Material with EY on Production Yield, Encapsulation Efficiency and Moist Heat Resistance of Microcapsules
3.3. Molecular Characteristics of Microcapsules
3.4. Effect of the Ratio of EY to AA on Morphology, Particle Size and Size Distribution of Microcapsules
3.5. Physical Properties and Stability of Pelleted Feeds in Seawater
3.5.1. Physical Properties of Pelleted Feeds
3.5.2. Stability of Pelleted Feeds in Seawater
3.5.3. Losses of Fortified AA during the Production Process of Feed Pellets and in Seawater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Hossain, M.S.; Moss, A.S. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture 2018, 491, 197–204. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Sánchez, D.R.; Fox, J.M.; Gatlin Iii, D.; Lawrence, A.L. Dietary effect of fish oil and soybean lecithin on growth and survival of juvenile L itopenaeus vannamei in the presence or absence of phytoplankton in an indoor system. Aquac. Res. 2014, 45, 1367–1379. [Google Scholar] [CrossRef]
- Truong, H.H.; Moss, A.F.; Bourne, N.A.; Simon, C.J. Determining the Importance of Macro and Trace Dietary Minerals on Growth and Nutrient Retention in Juvenile Penaeus monodon. Animals 2020, 10, 2086. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.M. Synthetic or food-derived vitamin C—Are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef]
- Niu, J.; Tian, L.X.; Liu, Y.J.; Mai, K.S.; Yang, H.J.; Ye, C.X.; Gao, W. Nutrient values of dietary ascorbic acid (L-ascorbyl-2-polyphosphate) on growth, survival and stress tolerance of larval shrimp, Litopenaeus vannamei. Aquac. Nutr. 2009, 15, 194–201. [Google Scholar] [CrossRef]
- Wang, W.-N.; Wang, Y.; Wang, A.-L. Effect of supplemental L-ascorbyl-2-polyphosphate (APP) in enriched live food on the immune response of Penaeus vannamei exposed to ammonia-N. Aquaculture 2006, 256, 552–557. [Google Scholar] [CrossRef]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M.; Cheruparambath, P. Effect of different levels dietary vitamin C on growth performance, muscle composition, antioxidant and enzyme activity of freshwater prawn, Macrobrachium malcolmsonii. Aquac. Rep. 2016, 3, 229–236. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, E.; Ruyra, A.; Roher, N.; Zuasti, E.; Infante, C.; Fernández-Díaz, C. Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture 2014, 432, 426–433. [Google Scholar] [CrossRef]
- Morin, P.; Gorman, A.; Lambrakis, L. A literature review on vitamin retention during the extrusion of dry pet food. Anim. Feed Sci. Technol. 2021, 277, 114975. [Google Scholar] [CrossRef]
- Masoomi Dezfooli, S.; Gutierrez-Maddox, N.; Alfaro, A.; Seyfoddin, A. Encapsulation for delivering bioactives in aquaculture. Rev. Aquac. 2019, 11, 631–660. [Google Scholar] [CrossRef]
- Yang, P.; Wang, H.; Zhu, M.; Ma, Y. Evaluation of Extrusion Temperatures, Pelleting Parameters, and Vitamin Forms on Vitamin Stability in Feed. Animals 2020, 10, 894. [Google Scholar] [CrossRef]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-drying performance and thermal stability of L-ascorbic acid microencapsulated with sodium alginate and gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Jafari, M.; Fazel, M. Potential of microencapsulation to protect ascorbic acid under different temperature and pH during heating process. Braz. J. Technol. 2019, 2, 573–589. Available online: https://brazilianjournals.com/ojs/index.php/BJT/article/view/2067/2211 (accessed on 6 September 2022).
- Marcet, I.; Sáez-Orviz, S.; Rendueles, M.; Díaz, M. Egg yolk granules and phosvitin. Recent advances in food technology and applications. LWT-Food Sci. Technol. 2022, 153, 112442. [Google Scholar] [CrossRef]
- Anton, M. Egg yolk: Structures, functionalities and processes. J. Sci. Food Agric. 2013, 93, 2871–2880. [Google Scholar] [CrossRef]
- Li, J.; Zhai, J.; Gu, L.; Su, Y.; Gong, L.; Yang, Y.; Chang, C. Hen egg yolk in food industry-A review of emerging functional modifications and applications. Trends Food Sci. Technol. 2021, 115, 12–21. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Q.; Wang, T.; Xue, J.; Luo, Y. Characterization of high density lipoprotein from egg yolk and its ability to form nanocomplexes with chitosan as natural delivery vehicles. Food Hydrocoll. 2018, 77, 204–211. [Google Scholar] [CrossRef]
- Zorriehzahra, M.J.; Tiwari, R.; Sachan, S.; Karthik, K.; Malik, Y.S.; Dadar, M.; Sarwar, M.; Sayab, M.; Dhama, K. Avian egg yolk antibodies (IgY) and their potential therapeutic applications for countering infectious diseases of fish and aquatic animals. Int. J. Pharmacol. 2016, 12, 760–768. [Google Scholar] [CrossRef]
- Kiosseoglou, V. Egg yolk protein gels and emulsions. Curr. Opin. Colloid Interface Sci. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Javed, A.; Imran, M.; Ahmad, N.; Hussain, A.I. Fatty acids characterization and oxidative stability of spray dried designer egg powder. Lipids Health Dis. 2018, 17, 282. [Google Scholar] [CrossRef] [PubMed]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: Effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J. Food Eng. 2018, 223, 91–98. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Shiu, Y.-L.; Chiu, S.-T.; Ballantyne, R.; Liu, C.-H. Effects of chitin from Daphnia similis and its derivative, chitosan on the immune response and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 119, 329–338. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.K.; Singh, S.P.; Awasthi, A. Immunostimulants for shrimp aquaculture: Paving pathway towards shrimp sustainability. Environ. Sci. Pollut. Res. 2022, 1–19. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.P.; Shivhare, U.S. Microencapsulation by complex coacervation using whey protein isolates and gum acacia: An approach to preserve the functionality and controlled release of β-carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Rout, R.K.; Bandyopadhyay, S. A comparative study of shrimp feed pellets processed through cooking extruder and meat mincer. Aquac. Eng. 1999, 19, 71–79. [Google Scholar] [CrossRef]
- Obaldo, L.G.; Divakaran, S.; Tacon, A.G. Method for determining the physical stability of shrimp feeds in water. Aquac. Res. 2002, 33, 369–377. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Howes, T. Implication of glass transition for the drying and stability of dried foods. J. Food Eng. 1999, 40, 71–79. [Google Scholar] [CrossRef]
- Bhandari, B.; Adhikari, B. Glass-transition based approach in drying of foods. In Advances in Food Dehydration; CRC Press: Boca Raton, FL, USA, 2008; pp. 37–62. [Google Scholar]
- Sartori, T.; Consoli, L.; Hubinger, M.D.; Menegalli, F.C. Ascorbic acid microencapsulation by spray chilling: Production and characterization. LWT-Food Sci. Technol. 2015, 63, 353–360. [Google Scholar] [CrossRef]
- Abbasnezhad, B.; Hamdami, N.; Monteau, J.Y.; Vatankhah, H. Numerical modeling of heat transfer and pasteurizing value during thermal processing of intact egg. Food Sci. Nutr. 2016, 4, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, J.S.R.; Gabas, A.L.; Minim, L.A.; Rojas, E.E.G.; Telis, V.R.N.; Telis-Romero, J. Density, heat capacity and thermal conductivity of liquid egg products. J. Food Eng. 2006, 74, 186–190. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, T.; Wei, H.; Dang, L. Stability of sodium ascorbyl phosphate in the water-glycerol system. J. Pharm. Biomed. Anal. 2020, 181, 113103. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.; Blaga, A.; Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. 2016, 289, 71–78. [Google Scholar] [CrossRef]
- Carvalho, A.G.S.; Silva, V.M.; Hubinger, M.D. Microencapsulation by spray drying of emulsified green coffee oil with two-layered membranes. Food Res. Int. 2014, 61, 236–245. [Google Scholar] [CrossRef]
- Yinbin, L.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of different encapsulating agent combinations on physicochemical properties and stability of microcapsules loaded with phenolics of plum (Prunus salicina lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar] [CrossRef]
- Fuertes, S.; Laca, A.; Oulego, P.; Paredes, B.; Rendueles, M.; Díaz, M. Development and characterization of egg yolk and egg yolk fractions edible films. Food Hydrocoll. 2017, 70, 229–239. [Google Scholar] [CrossRef]
- Ganesan, P.; Benjakul, S.; Baharin, B.S. Effect of different cations in pickling solution on FTIR characteristics of pidan white and yolk in Comparison to the fresh duck egg. Sains Malays. 2014, 43, 1883–1887. [Google Scholar] [CrossRef]
- Michalczyk, E.; Kurczab, R. Assessment of poultry eggs freshness using FTIR spectroscopy combined with HCA and PCA methods. Sci. Technol. Innov. 2018, 2, 7–12. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-raman and SERS spectra of vitamin C. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of copper nanoparticles using L-ascorbic acid. Matéria 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Ahmed, I.; Haque, A.; Bhattacharyya, S.; Patra, P.; Plaisier, J.R.; Perissinotto, F.; Bal, J.K. Vitamin C/Stearic Acid Hybrid Monolayer Adsorption at Air–Water and Air–Solid Interfaces. ACS Omega 2018, 3, 15789–15798. [Google Scholar] [CrossRef] [Green Version]





| Ingredients | Composition of Shrimp Feed Sample (g/100 g Feed) | ||
|---|---|---|---|
| Plain Pelleted Feed | Pelleted Feed Containing Unencapsulated AA | Pelleted Feed Containing Encapsulated AA | |
| Cereal | 40.00 | 39.92 | 39.80 |
| Soybean meal | 30.00 | 29.94 | 29.85 |
| Fishmeal | 12.00 | 11.98 | 11.94 |
| Shrimp head meal | 10.00 | 9.98 | 9.95 |
| Fish oil | 4.00 | 3.98 | 3.98 |
| Dicalcium phosphate | 2.00 | 2.00 | 1.99 |
| Corn starch | 2.00 | 2.00 | 1.99 |
| AA | 0 | 0.20 | 0 |
| Microcapsule E1A1 (AA payload in microcapsules = 40%) | 0 | 0 | 0.50 * |
| Total | 100.00 | 100.00 | 100.00 |
| Physical Properties | Plain Pelleted Feed | Pelleted Feed Containing Unencapsulated AA | Pelleted Feed Containing Encapsulated AA | Commercial Pelleted Feed |
|---|---|---|---|---|
| Moisture content (d.b., %) | 11.03 ± 0.67 a | 10.98 ± 0.41 a | 11.21 ± 0.33 a | 11.28 ± 0.42 a |
| Bulk density (g/cm3) | 0.52 ± 0.01 b | 0.56 ± 0.02 b | 0.53 ± 0.02 b | 0.63 ± 0.01 a |
| Sinking velocity (cm/s) | 4.21 ± 0.78 b | 4.49 ± 0.42 b | 4.31 ± 0.46 b | 5.01 ± 0.38 a |
| Process | Retention of AA in Pelleted Feed (%) | |
|---|---|---|
| Unencapsulated AA | Encapsulated AA | |
| Before extrusion | 100.0 | 100.0 |
| After extrusion | 33.7 | 85.8 |
| After drying at 90 °C for 1 h | 17.4 | 66.6 |
| After exposure to seawater for 1 h | 12.3 | 63.5 |
| After exposure to seawater for 3 h | 8.1 | 57.6 |
| After exposure to seawater for 6 h | 3.0 | 46.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroensaensuai, J.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S.; Rungrassamee, W. Improvement of Moist Heat Resistance of Ascorbic Acid through Encapsulation in Egg Yolk–Chitosan Composite: Application for Production of Highly Nutritious Shrimp Feed Pellets. Animals 2022, 12, 2384. https://doi.org/10.3390/ani12182384
Jaroensaensuai J, Wongsasulak S, Yoovidhya T, Devahastin S, Rungrassamee W. Improvement of Moist Heat Resistance of Ascorbic Acid through Encapsulation in Egg Yolk–Chitosan Composite: Application for Production of Highly Nutritious Shrimp Feed Pellets. Animals. 2022; 12(18):2384. https://doi.org/10.3390/ani12182384
Chicago/Turabian StyleJaroensaensuai, Jidapa, Saowakon Wongsasulak, Tipaporn Yoovidhya, Sakamon Devahastin, and Wanilada Rungrassamee. 2022. "Improvement of Moist Heat Resistance of Ascorbic Acid through Encapsulation in Egg Yolk–Chitosan Composite: Application for Production of Highly Nutritious Shrimp Feed Pellets" Animals 12, no. 18: 2384. https://doi.org/10.3390/ani12182384