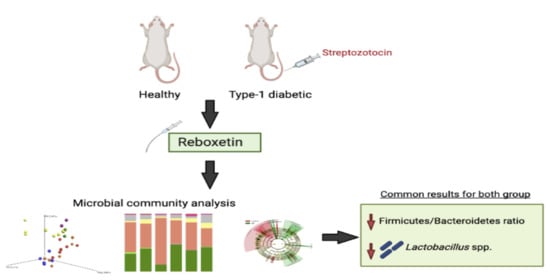
Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Induction of Experimental Diabetes
2.4. Measurement of Blood Glucose Levels
2.5. Experimental Groups
2.6. DNA Isolation and Library Preparation
2.7. Microbial Community Analysis
2.8. Statistical Analysis
3. Results
3.1. Microbial Community Differences between the Groups
3.2. Relative Taxa Abundances in Phylum Level between the Groups
3.3. Differential Taxa in Diabetic and Reboxetine-Treated Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackermann, R.T.; Rosenman, M.B.; Downs, S.M.; Holmes, A.M.; Katz, B.P.; Li, J.; Zilich, A.J.; Carney, C.P.; Inui, T.S. Telephonic case-finding of major depression in a Medicaid chronic disease management program for diabetes and heart failure. Gen. Hosp. Psychiatry 2005, 27, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G.; de Groot, M.; Golden, S.H. Diabetes and depression. Curr. Diab. Rep. 2014, 14, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, P.; Li, Z.; Zhou, Z. Gut microbiome in type 1 diabetes: A comprehensive review. Diabetes/Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef]
- Eyding, D.; Lelgemann, M.; Grouven, U.; Härter, M.; Kromp, M.; Kaiser, T.; Kerekes, M.F.; Gerken, M.; Wieseler, B. Reboxetine for acute treatment of major depression: Systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor-controlled trials. BMJ 2010, 341, c4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajós, M.; Fleishaker, J.C.; Filipiak-Reisner, J.K.; Brown, M.T.; Wong, E.H.F. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: Pharmacological and clinical profile. CNS Drug Rev. 2004, 10, 23–44. [Google Scholar] [CrossRef]
- Turan, Y.N.; Can, Ö.D.; Özkay, Ü.D. Catecholaminergic and opioidergic system mediated effects of reboxetine on diabetic neuropathic pain. Psychopharmacology 2020, 237, 1131–1145. [Google Scholar] [CrossRef]
- Wong, E.H.; Sonders, M.S.; Amara, S.G.; Tinholt, P.M.; Piercey, M.F.; Hoffman, W.P.; Hyslop, D.K.; Franklin, S.; Porsolt, R.D.; Bonsignori, A.; et al. Reboxetine: A pharmacologically potent, selective and specific norepinephrine reuptake inhibitor. Biol. Psychiatry 2020, 47, 818–829. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Emmanuelle, C.; Sunaggawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Yang, C.; Qu, Y.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 2017, 7, 1294. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Fu, J.; Ma, S.R.; Peng, R.; Yu, J.B.; Cong, L.; Pan, L.B.; Zhang, Z.G.; Tian, H.; Che, C.T.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomassetti, C.; et al. Gut microbiota and bipolar disorder: An overview on a novel biomarker for diagnosis and treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, S.; Go, M.J.; Lee, S.Y.; Kim, S.C.; Lee, C.H.; Byung-Kwan, C. Analysis of the mouse gut microbiome using full-length 16S RNA amplicon sequencing. Sci. Rep. 2016, 6, 29681. [Google Scholar] [CrossRef] [Green Version]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 2, e7502. [Google Scholar] [CrossRef] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2019, 51, 203590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkul, C.; Ruiz, V.E.; Battaglia, T.; Xu, J.; Roubaud-Baudron, C.; Cadwell, K.; Guillermo, I.P.P.; Blaser, M.J. A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis. Genome Med. 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Yan, J.E.; Yuan, W.; Lou, X.; Zhu, T. Streptozotocin-induced diabetic hyperalgesia in rats is associated with upregulation of Toll-like receptor 4 expression. Neurosci. Lett. 2012, 526, 54–58. [Google Scholar] [CrossRef]
- Khanam, R.; Pillai, K.K. Lack of hypo/hyperglycemic effects of reboxatine in dibetic and non-diabetic rats. Fundam. Clin. Pharmacol. 2005, 19, 657–659. [Google Scholar] [CrossRef]
- Aydın, T.H.; Can, Ö.D.; Özkay, Ü.D.; Turan, N. Effect of subacute agomelatine treatment on painful diabetic neuropathy: Involvement of catecholaminergic mechanisms. Fundam. Clin. Pharm. 2016, 30, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Barbaros, M.B.; Can, Ö.D.; Üçel, U.İ.; Yücel, N.T.; Özkay, Ü.D. Antihyperalgesic activity of atomoxetine on diabetes-induced neuropathic pain: Contribution of noradrenergic and dopaminergic systems. Molecules 2018, 23, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtake, K.; Ishiyama, Y.; Uchida, H.; Muraki, E.; Kobayashi, J. Dietary nitrite inhibits early glomerular injury in streptozotocin-induced diabetic nephropathy in rats. Nitric Oxide 2007, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Skalska, S.; Kyselova, Z.; Gajdosikova, A.; Karasu, C.; Stefek, M.; Stolc, S. Protective effect of stobadine on NCV in streptozotocin-diabetic rats: Augmentation by vitamin E. Gen. Physiol. Biophys. 2008, 27, 106–114. [Google Scholar] [PubMed]
- Cegielska-Perun, K.; Bujalska-Zadrożny, M.; Gąsińska, E.; Makulska-Nowak, H.E. Enhancement of antinociceptive effect of morphine by antidepressants in diabetic neuropathic pain model. Pharmacol. Rep. 2014, 66, 228–234. [Google Scholar] [CrossRef]
- Pedersen, L.H.; Nielsen, A.N.; Blackburn-Munro, G. Anti-nociception is selectively enhanced by parallel inhibition of multiple subtypes of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology 2005, 182, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Bektur, E.; Donmez, D.B.; Baycu, C.; Can, O.D.; Sahinturk, V. Mirtazapine suppresses sterile inflammation through NLRP3-inflammasome in diabetic rat kidney. Acta Histochem. 2019, 121, 289–296. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goosrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Lu, J.; Zhang, X. Metabolic Influences of commonly used antidepressants on blood glucose homeostasis. Indian J. Pharm. Sci. 2019, 81, 188–199. [Google Scholar] [CrossRef]
- Markowitz, S.M.; Gonzalez, J.S.; Wilkinson, J.L.; Safren, S.A. A review of treating depression in diabetes: Emerging findings. Psychosomatics 2011, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ozkul, C.; Yalinay, M.; Karakan, T. Structural changes in gut microbiome after ramadan fasting: A pilot study. Benef. Microbes 2020, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Roubaud-Baudron, C.; Ruiz, V.E.; Swan, A.M.; Vallance, B.A.; Ozkul, C.; Pei, Z.; Li, J.; Battaglia, T.W.; Perez-Perez, G.I.; Blasern, M.J. Long-term effects of early-life antibiotic exposure on resistance to subsequent bacterial infection. Am. Soc. Microbiol. 2019, 24, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vich-Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of comonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Paslakis, G.; Gilles, M.; Ledeebogen, F.; Schilling, C.; Scharnholz, B.; Deuschle, M. The effect of a 4-week treatment with reboxetine on metabolic parameters of depressed inpatients. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Amrami-Weizman, A.; Maayan, R.; Gil-Ad, I.; Pashinian, A.; Fuchs, C.; Kotler, M.; Poyurovsky, M. The effect of reboxetine co-administration with olanzapine on metabolic and endocrine profile in schizophrenia patients. Pschopharmacology 2013, 230, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, 8–21. [Google Scholar] [CrossRef]
- Patterson, E.; Marques, T.M.; O’Sullivan, O.; Fitzgerald, P.; Fitzgerald, G.F.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F.; Stanton, C.; Ross, R.P. Streptozotocin-induced type-1-diabetes disease onset in Sprague–Dawley rats is associated with an altered intestinal microbiota composition and decreased diversity. Microbiology 2015, 161, 182–193. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Jenewein, I.; Ledochowski, M. Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J. Antimicrob. Chemoth. 2001, 48, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Surono, I.S.; Wardana, A.A.; Waspodo, P.; Saksono, B.; Verhoeven, J.; Venema, K. Effect of functional food ingredients on gut microbiota in a rodent diabetes model. Nutr. Metab. 2020, 17, 77. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Miele, L.; Vananzio, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Wirth, R.; Bódi, N.; Maróti, G.; Bagyánszki, M.; Talapka, P.; Fekete, É.; Bagi, Z. Regionally distinct alterations in the composition of the gut microbiota in rats with Streptozotocin-Induced diabetes. PLoS ONE 2014, 9, e110440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common factor in human diseases. Biomed. Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganism 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Gut microbiota and obesity: Lessons from the microbiome. Brief. Funct. Genom. 2013, 12, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.R.; Zhou, L.Z.; Fang, S.T.; Long, H.Y.; Chen, J.Y.; Zhang, G.X. Isolation of Desulfovibrio spp. from human gut microbiota using a next- generation sequencing directed culture method. Lett. Appl. Microbiol. 2019, 68, 553–561. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Raghunanthan, K.; Campbell, A.M.; Kim, S.W.; Lau, K.S.; Kenworthy, A.K.; Cover, T.L.; Ohi, M.D. Intracellular degradation of Helicobacter pylori VacA toxin as a determinant of gastric epithelial cell viability. Infect. Immun. 2019, 87, e00783-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; De la Fuente, M.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing thr bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Kim, N.; Nam, R.H.; Park, J.H.; Choi, S.; Park, T.-T.; Kim, Y.-R.; Seok, Y.-J.; Shin, C.M.; Lee, D.H. Gut microbiota and butyrate level changes associated with the long-term administration of proton pump inhibitors to old rats. Sci. Rep. 2019, 9, 6626. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alrefai, W.A.; Borthakur, A.; Dudeja, P.K. Lactobacillus acidophilus counteracts enteropathogenic E.coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am. J. Phtsiol. 2015, 309, G602–G607. [Google Scholar]
- McNeil, N.I.; Cummings, J.H.; James, W.P. Short chain fatty acid absorption by the human large intestine. Gut 1978, 19, 819–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Denan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Allini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.T.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403–1417. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, J.; Yao, W.; Ren, Q.; Yang, C.; Ma, M.; Han, M.; Saito, R.; Hashimoto, K. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2016, 144, 7–12. [Google Scholar] [CrossRef]
- Vasseur, M.V.; Laurentie, M.; Rolland, J.G.; Perrin-Guyomard, A.; Henri, J.; Ferran, A.A.; Toutain, P.L.; Bousquet-Melou, A. Low or high doses of cefquinome targeting low or high bacterial inocula cure Klebsiella pneumoniae lung infections but differentially impact the levels of antibiotic resistance in fecal flora. Antimicrob. Agents Chemother. 2014, 58, 1744–1748. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Jarvenpaa, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Berardis, D.; Conti, C.M.V.; Serroni, N.; Moschetta, F.S.; Olivieri, L.; Carano, A.; Salerno, R.M.; Cavuto, M.; Farina, B.; Allessandrini, M.; et al. The effect of newer serotonin-noradrenalın antidepressant on cytokıne production: A review of the current literrature. Int. J. Immunopathol. 2010, 23, 417–422. [Google Scholar] [CrossRef]
- Galecki, P.; Mossakowska-Wojcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressant-SSRIs, SNRIs. Prog. Neuropsychopharmacol. Biol. Pschiatry 2018, 80, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Sharma, B.; Timmons, B.W. The efficiency of anti-inflammatory tratment interventions on depression in individuals with major depressive disorder and high level of inflammation: A systematic review of randomşzed clinical trials. Physiol. Behav. 2019, 207, 104–112. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, S.; Ozkul, C.; Yucel, N.T.; Karaca, H. Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats. Microorganisms 2021, 9, 1948. https://doi.org/10.3390/microorganisms9091948
Aydin S, Ozkul C, Yucel NT, Karaca H. Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats. Microorganisms. 2021; 9(9):1948. https://doi.org/10.3390/microorganisms9091948
Chicago/Turabian StyleAydin, Sinem, Ceren Ozkul, Nazlı Turan Yucel, and Hulya Karaca. 2021. "Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats" Microorganisms 9, no. 9: 1948. https://doi.org/10.3390/microorganisms9091948