Tick Fauna and Associated Rickettsia, Theileria, and Babesia spp. in Domestic Animals in Sudan (North Kordofan and Kassala States)
Abstract
:1. Introduction
2. Materials and Methods
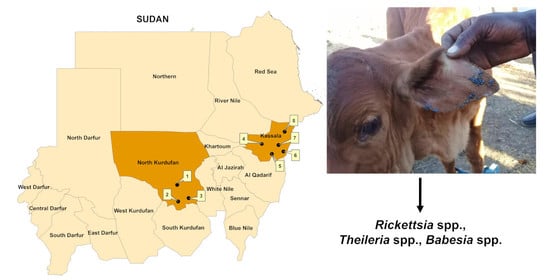
2.1. Study Area
2.2. Tick Collection and Identification
2.3. Nucleic Acid Extraction
2.4. Molecular Tick Species Identification
2.5. PCR for Rickettsia spp. and Piroplasms
3. Results
3.1. Identified Tick Species
3.2. Prevalence of Tick-Borne Pathogens
3.2.1. Rickettsia Species
3.2.2. Piroplasms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uilenberg, G. Veterinary significance of ticks and tick-borne diseases. In Tick Vector Biology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 23–33. [Google Scholar]
- Obregón Alvarez, D.; Corona-González, B.; Rodríguez-Mallón, A.; Rodríguez Gonzalez, I.; Alfonso, P.; Noda Ramos, A.A.; Díaz-Sánchez, A.A.; González Navarrete, M.; Rodríguez Fernández, R.; Méndez Mellor, L.; et al. Ticks and tick-borne diseases in Cuba, half a century of scientific research. Pathogens 2020, 9, 616. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [Green Version]
- Lew-Tabor, A.E.; Rodriguez Valle, M. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick Borne Dis. 2016, 7, 573–585. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J. Vital signs: Trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial nature of tick-borne diseases. mBio 2019, 10, e02055-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhanguzi, D.; Byaruhanga, J.; Amanyire, W.; Ndekezi, C.; Ochwo, S.; Nkamwesiga, J.; Mwiine, F.N.; Tweyongyere, R.; Fourie, J.; Madder, M.; et al. Invasive cattle ticks in East Africa: Morphological and molecular confirmation of the presence of Rhipicephalus microplus in south-eastern Uganda. Parasites Vectors 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makenov, M.; Toure, A.; Korneev, M.; Sacko, N.; Porshakov, A.; Yakovlev, S.; Radyuk, E.; Zakharov, K.; Shipovalov, A.; Boumbaly, S.; et al. Rhipicephalus microplus and its vector-borne haemoparasites in Guinea: Further species expansion in West Africa. bioRxiv 2020. [Google Scholar] [CrossRef]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Endinburgh, UK, 2014. [Google Scholar]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites Vectors 2016, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Raboloko, O.O.; Ramabu, S.S.; Guerrini, L.; Jori, F. Seroprevalence of selected tick borne pathogens and diversity and abundance of ixodid ticks (Acari: Ixodidae) at the wildlife-livestock interface in Northern Botswana. Front. Vet. Sci. 2020, 7, 187. [Google Scholar] [CrossRef]
- Shuaib, Y.A.; Osman, H.M.; Hussein, M.O.; Bakhiet, M.A.; Omer, R.A.; Al-Nahas, A.; Suliman, S.E.; Abdalla, M.A.; Ismail, A.A. Seroprevalence of Babesia bigemina antibodies in cattle in North Kordofan state, the Sudan. ARC J. Anim. Vet. Sci. 2015, 1, 1–11. [Google Scholar]
- Chitimia-Dobler, L.; Issa, M.H.; Ezalden, M.E.; Yagoub, I.A.; Abdalla, M.A.; Bakhiet, A.O.; Schaper, S.; Rieß, R.; Vollmar, P.; Grumbach, A.; et al. Crimean-Congo haemorrhagic fever virus in Hyalomma impeltatum ticks from North Kordofan, the Sudan. Int. J. Infect. Dis. 2019, 89, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuaib, Y.A.; Elhag, A.M.-A.W.; Brima, Y.A.; Abdalla, M.A.; Bakiet, A.O.; Mohmed-Noor, S.E.-T.; Lemhöfer, G.; Bestehorn, M.; Poppert, S.; Schaper, S.; et al. Ixodid tick species and two tick-borne pathogens in three areas in the Sudan. Parasitol. Res. 2020, 119, 385–394. [Google Scholar] [CrossRef] [PubMed]
- MARF. Number of Animals in the Sudan; Ministry of Animal Resources and Fisheries (MARF), Statistics: Khartoum, Sudan, 2012.
- Hassan, S.; Salih, D. An overview of factors responsible for geographic distribution pattern of ixodid ticks in the Sudan. Sokoto J. Vet. Sci. 2013, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Salih, D.A.; El Hussein, A.M.; Seitzer, U.; Ahmed, J.S. Epidemiological studies on tick-borne diseases of cattle in Central Equatoria State, Southern Sudan. Parasitol. Res. 2007, 101, 1035–1044. [Google Scholar] [CrossRef]
- Salim, B.; Bakheit, M.A.; Kamau, J.; Sugimoto, C. Current status of equine piroplasmosis in the Sudan. Infect. Genet. Evol. 2013, 16, 191–199. [Google Scholar] [CrossRef]
- Nakao, M.; Qiu, Y.; Salim, B.; Hassan, S.M.; Sugimoto, C. Molecular detection of Rickettsia africae in Amblyomma variegatum collected from Sudan. Vector Borne Zoonotic Dis. 2015, 15, 323–325. [Google Scholar] [CrossRef] [Green Version]
- Maina, A.N.; Jiang, J.; Omulo, S.A.; Cutler, S.J.; Ade, F.; Ogola, E.; Feikin, D.R.; Njenga, M.K.; Cleaveland, S.; Mpoke, S.; et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: Implications for human health. Vector Borne Zoonotic Dis. 2014, 14, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Apanaskevich, D.; Horak, I. The genus Hyalomma Koch, 1844: II Taxonomic status of H. (Euhyalomma) anatolicum Koch, 1844 and H. (E.) excavatum Koch, 1844 (Acari: Ixodidae) with re-description of all stages. Acarina 2005, 13, 181–197. [Google Scholar]
- Apanaskevich, D.A.; Horak, I.G. The genus Hyalomma Koch, 1844: V Re-evaluation of the taxonomic rank of taxa comprising the H. (Euhyalomma) marginatum Koch complex of species (Acari: Ixodidae) with redescription of all parasitic stages and notes on biology. Int. J. Acarol. 2008, 34, 13–42. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Horak, I.G. The genus Hyalomma Koch, 1844. IX. Redescription of all parasitic stages of H. (Euhyalomma) impeltatum Schulze & Schlottke, 1930 and H. (E.) somalicum Tonelli Rondelli, 1935 (Acari: Ixodidae). Syst. Parasitol. 2009, 73, 199–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apanaskevich, D.A.; Schuster, A.L.; Horak, I.G. The genus Hyalomma: VII. Redescription of all parasitic stages of H. (Euhyalomma) dromedarii and H. (E.) schulzei (Acari: Ixodidae). J. Med. Entomol. 2008, 45, 817–831. [Google Scholar] [CrossRef]
- Voltzit, O.; Keirans, J. A review of African Amblyomma species (Acari, Ixodida, Ixodidae). Acarina 2003, 11, 135–214. [Google Scholar]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol. Res. 1998, 84, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Bakkes, D.K.; Chitimia-Dobler, L.; Matloa, D.; Oosthuysen, M.; Mumcuoglu, K.Y.; Mans, B.J.; Matthee, C.A. Integrative taxonomy and species delimitation of Rhipicephalus turanicus (Acari: Ixodida: Ixodidae). Int. J. Parasitol. 2020, 50, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [Green Version]
- Chitimia-Dobler, L.; Langguth, J.; Pfeffer, M.; Kattner, S.; Küpper, T.; Friese, D.; Dobler, G.; Guglielmone, A.A.; Nava, S. Genetic analysis of Rhipicephalus sanguineus sensu lato ticks parasites of dogs in Africa north of the Sahara based on mitochondrial DNA sequences. Vet. Parasitol. 2017, 239, 1–6. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Latrofa, M.; Annoscia, G.; Giannelli, A.; Parisi, A.; Otranto, D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors 2013, 6, 213. [Google Scholar] [CrossRef] [Green Version]
- Nava, S.; Beati, L.; Venzal, J.M.; Labruna, M.B.; Szabó, M.P.J.; Petney, T.; Saracho-Bottero, M.N.; Tarragona, E.L.; Dantas-Torres, F.; Silva, M.M.S.; et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick. Borne. Dis. 2018, 9, 1573–1585. [Google Scholar] [CrossRef]
- Sands, A.F.; Apanaskevich, D.A.; Matthee, S.; Horak, I.G.; Harrison, A.; Karim, S.; Mohammad, M.K.; Mumcuoglu, K.Y.; Rajakaruna, R.S.; Santos-Silva, M.M.; et al. Effects of tectonics and large scale climatic changes on the evolutionary history of Hyalomma ticks. Mol. Phylogenet. Evol. 2017, 114, 153–165. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.-I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, B.; Chamberlain, J.; Logue, C.H.; Cook, N.; Bruce, C.; Dowall, S.D.; Hewson, R. Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis. 2012, 12, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Essbauer, S.; Dobler, G. Diagnostics of tick-borne rickettsioses in Germany: A modern concept for a neglected disease. Int. J. Med. Microbiol. 2008, 298, 368–374. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Riess, R.; Kahl, O.; Wolfel, S.; Dobler, G.; Nava, S.; Estrada-Pena, A. Ixodes inopinatus—Occurring also outside the Mediterranean region. Ticks Tick Borne Dis. 2018, 9, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, L.; Chelo, I.M.; Bacellar, F.; Zé-Zé, L. Rickettsiae phylogeny: A multigenic approach. Microbiology 2007, 153, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Weinert, L.A.; Werren, J.H.; Aebi, A.; Stone, G.N.; Jiggins, F.M. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009, 7, 6. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar]
- Springer, A.; Höltershinken, M.; Lienhart, F.; Ermel, S.; Rehage, J.; Hülskötter, K.; Lehmbecker, A.; Wohlsein, P.; Barutzki, D.; Gietl, C.; et al. Emergence and epidemiology of bovine babesiosis due to Babesia divergens on a northern German beef production farm. Front. Vet. Sci. 2020, 7, 649. [Google Scholar] [CrossRef]
- Adehan, S.B.; Adakal, H.; Gbinwoua, D.; Yokossi, D.; Zoungrana, S.; Toe, P.; Ouedraogo, M.; Gbaguidi, A.M.; Adoligbé, C.; Fandohan, A.B.; et al. West African cattle farmers’ perception of tick-borne diseases. EcoHealth 2018, 15, 437–449. [Google Scholar] [CrossRef]
- Jongejan, F.; Zivkovic, D.; Pegram, R.G.; Tatchell, R.J.; Fison, T.; Latif, A.A.; Paine, G. Ticks (Acari:Ixodidae) of the Blue and White Nile ecosystems in the Sudan with particular reference to the Rhipicephalus sanguineus group. Exp. Appl. Acarol. 1987, 3, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Karrar, G.; Kaiser, M.N.; Hoogstraal, H. Ecology and host-relationships of ticks (Ixodoidea) infesting domestic animals in Kassala Province, Sudan, with special reference to Amblyomma lepidum Dönitz. Bull. Entomol. Res. 1963, 54, 509–522. [Google Scholar] [CrossRef]
- Salih, D.A.; Hassan, S.M.; El Hussein, A.M.; Jongejan, F. Preliminary survey of ticks (Acari: Ixodidae) on cattle in northern Sudan. Onderstepoort J. Vet. Res. 2004, 71, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed-Ahmed, G.M.; Hassan, S.M.; El Hussein, A.M.; Salih, D.A. Molecular, serological and parasitological survey of Theileria annulata in North Kordofan State, Sudan. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.M.; El-Hussein, A.M.; El-Khider, A.O. Some observations on ticks (Acari: Ixodidae) infesting sheep in River Nile Province of Northern Sudan. Onderstepoort J. Vet. Res. 2005, 72, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Elghali, A.; Hassan, S.M. Ticks (Acari: Ixodidae) infesting camels (Camelus dromedarius) in Northern Sudan. Onderstepoort J. Vet. Res. 2009, 76, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, I.; Holzmuller, P.; Stachurski, F.; Rodrigues, V.; Vachiéry, N. Ehrlichia ruminantium: The causal agent of heartwater. In Rickettsiales: Biology, Molecular Biology, Epidemiology, and Vaccine Development; Thomas, S., Ed.; Springer: Cham, Switzerland, 2016; pp. 241–280. [Google Scholar] [CrossRef]
- Balinandi, S.; Chitimia-Dobler, L.; Grandi, G.; Nakayiki, T.; Kabasa, W.; Bbira, J.; Lutwama, J.J.; Bakkes, D.K.; Malmberg, M.; Mugisha, L. Morphological and molecular identification of ixodid tick species (Acari: Ixodidae) infesting cattle in Uganda. Parasitol. Res. 2020, 119, 2411–2420. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [Green Version]
- Kumsa, B.; Socolovschi, C.; Raoult, D.; Parola, P. Spotted fever group rickettsiae in ixodid ticks in Oromia, Ethiopia. Ticks Tick Borne Dis. 2015, 6, 8–15. [Google Scholar] [CrossRef]
- Morita, C.; El Hussein, A.R.; Matsuda, E.; Abdel Gabbar, K.M.; Muramatsu, Y.; Abdel Rahman, M.B.; Eleragi, A.M.; Hassan, S.M.; Chitambo, A.M.; Ueno, H. Spotted fever group rickettsiae from ticks captured in Sudan. Jpn. J. Infect. Dis. 2004, 57, 107–109. [Google Scholar]
- Mura, A.; Socolovschi, C.; Ginesta, J.; Lafrance, B.; Magnan, S.; Rolain, J.-M.; Davoust, B.; Raoult, D.; Parola, P. Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Znazen, A.; Khrouf, F.; Elleuch, N.; Lahiani, D.; Marrekchi, C.; M’Ghirbi, Y.; Ben Jemaa, M.; Bouattour, A.; Hammami, A. Multispacer typing of Rickettsia isolates from humans and ticks in Tunisia revealing new genotypes. Parasites Vectors 2013, 6, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamani, J.; Baneth, G.; Mumcuoglu, K.Y.; Waziri, N.E.; Eyal, O.; Guthmann, Y.; Harrus, S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl. Trop. Dis. 2013, 7, e2108. [Google Scholar] [CrossRef] [PubMed]
- Mutai, B.K.; Wainaina, J.M.; Magiri, C.G.; Nganga, J.K.; Ithondeka, P.M.; Njagi, O.N.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. Zoonotic surveillance for rickettsiae in domestic animals in Kenya. Vector Borne Zoonotic Dis. 2013, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, G.; Killmaster, L.F.; Mumcuoglu, K.Y.; Levin, M.L. Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 2010, 52, 383–392. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Jongejan, F. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef]
- Horak, I.G.; Fourie, L.J.; Heyne, H.; Walker, J.B.; Needham, G.R. Ixodid ticks feeding on humans in South Africa: With notes on preferred hosts, geographic distribution, seasonal occurrence and transmission of pathogens. Exp. Appl. Acarol. 2002, 27, 113–136. [Google Scholar] [CrossRef]
- Eisawi, N.M.; Hassan, D.A.; Hussien, M.O.; Musa, A.B.; El Hussein, A.R.M. Seroprevalence of spotted fever group (SFG) rickettsiae infection in domestic ruminants in Khartoum State, Sudan. Vet. Med. Sci. 2017, 3, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Taha, K.M.; Salih, D.A.; Ali, A.M.; Omer, R.A.; El Hussein, A.M. Naturally occurring infections of cattle with Theileria lestoquardi and sheep with Theileria annulata in the Sudan. Vet. Parasitol. 2013, 191, 143–145. [Google Scholar] [CrossRef]
- El Hussein, A.M.; Hassan, S.M.; Salih, D.A. Current situation of tropical theileriosis in the Sudan. Parasitol. Res. 2012, 111, 503–508. [Google Scholar] [CrossRef]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.S.; Estrada-Peña, A.; Zintl, A. Vectors of Babesiosis. Ann. Rev. Entomol. 2019, 64, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Ma, M.; Moreau, E.; Liu, J.; Lu, B.; Bai, Q.; Luo, J.; Jorgensen, W.; Chauvin, A.; Yin, H. A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum. Exp. Parasitol. 2009, 122, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, M.C.; Allsopp, B.A.; Troskie, M.; Collins, N.E.; Penzhorn, B.L. Identification of novel Babesia and Theileria species in South African giraffe (Giraffa camelopardalis, Linnaeus, 1758) and roan antelope (Hippotragus equinus, Desmarest 1804). Vet. Parasitol. 2009, 163, 39–46. [Google Scholar] [CrossRef]
- Jouglin, M.; Fernández-de-Mera, I.G.; de la Cotte, N.; Ruiz-Fons, F.; Gortázar, C.; Moreau, E.; Bastian, S.; de la Fuente, J.; Malandrin, L. Isolation and characterization of Babesia pecorum sp. nov. from farmed red deer (Cervus elaphus). Vet. Res. 2014, 45, 78. [Google Scholar] [CrossRef] [Green Version]





| Rickettsia spp. | Babesia spp. | Theileria spp. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tick Species | Total No. of Ticks (% of All Ticks) | Females | Males | Nymphs | No. of Pools | Recorded Host Species | No. of Positive Pools | MIR 1 (%) | No. of Positive Pools | MIR 1 (%) | No. of Positive Pools | MIR 1 (%) |
| Genus Hyalomma | ||||||||||||
| Hyalomma impeltatum | 600 (24.9) | 340 | 260 | 0 | 97 | camel, cattle, sheep, goat, horse | 19 | 3.17 | 2 | 0.33 | 4 | 0.67 |
| Hyalomma anatolicum | 185 (7.68) | 71 | 70 | 44 | 89 | camel, cattle, sheep, goat, horse, dog | 4 | 2.16 | 0 | 0.00 | 4 | 2.16 |
| Hyalomma dromedarii | 136 (5.64) | 65 | 71 | 0 | 64 | camel, cattle, sheep | 6 | 4.41 | 0 | 0.00 | 0 | 0.00 |
| Hyalomma rufipes | 71 (2.95) | 16 | 55 | 0 | 32 | camel, cattle, sheep, goat | 13 | 18.31 | 0 | 0.00 | 0 | 0.00 |
| Hyalomma truncatum | 6 (0.25) | 1 | 5 | 0 | 2 | cattle | 2 | 33.33 | 0 | 0.00 | 0 | 0.00 |
| Genus Amblyomma | ||||||||||||
| Amblyomma lepidum | 387 (16.06) | 99 | 280 | 8 | 115 | cattle, sheep | 48 | 12.40 | 0 | 0.00 | 1 | 0.26 |
| Amblyomma variegatum | 58 (2.41) | 0 | 58 | 0 | 6 | cattle | 6 | 10.34 | 1 | 1.72 | 0 | 0.00 |
| Genus Rhipicephalus | ||||||||||||
| Rhipicephalus evertsi evertsi | 454 (18.84) | 168 | 286 | 0 | 191 | camel, cattle, sheep, goat, horse, dog | 10 | 2.20 | 0 | 0.00 | 4 | 0.88 |
| Rhipicephalus camicasi | 301 (12.49) | 164 | 137 | 0 | 128 | camel, cattle, sheep, goat, dog | 14 | 4.65 | 0 | 0.00 | 1 | 0.33 |
| Rhipicephalus decoloratus | 120 (5.00) | 120 | 0 | 0 | 23 | cattle, sheep, dog | 13 | 10.83 | 0 | 0.00 | 0 | 0.00 |
| Rhipicephalus sanguineus s.l. tropical lineage | 89 (3.69) | 33 | 56 | 0 | 33 | cattle, sheep, dog | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Rhipicephalus microplus | 2 (0.08) | 2 | 0 | 0 | 2 | cattle | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Rhipicephalus afranicus | 1 (0.04) | 1 | 0 | 0 | 1 | sheep | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 |
| Total | 2410 | 1080 | 1287 | 52 | 783 | 136 | 5.64 | 3 | 0.12 | 14 | 0.58 | |
| Piroplasm | Best Matches in GenBank | Sequence Identity (%), Query Cover (%) | Tick Species | MIR (Positive Pools/ Total Ticks) | State |
|---|---|---|---|---|---|
| Theileria ovis | MN712508, MN907458, MN704656 | 99–100, 99–100 | Hyalomma impeltatum | 0.67% (4/600) | North Kordofan |
| Hyalomma anatolicum | 0.54% (1/185) | North Kordofan | |||
| Rhipicephalus evertsi evertsi | 0.66% (3/454) | North Kordofan, Kassala | |||
| Rhipicephalus sanguineus | 0.34% (1/293) | North Kordofan | |||
| Theileria annulata | MN960099, MN907457, MK300062 | 100, 100 | Hyalomma anatolicum | 1.12% (1/89) | Kassala |
| Rhipicephalus evertsi evertsi | 0.22% (1/454) | Kassala | |||
| Theileria equi | MN625898, MN611343, MN611344 | 100, 99 | Hyalomma anatolicum | 0.54% (1/185) | Kassala |
| Theileria lestoquardi | MN704657, KY352037, KP793689 | 100, 100 | Hyalomma anatolicum | 0.54% (1/185) | Kassala |
| Theileria velifera | LC431550, MH424329, KY450754 | 100, 100 | Amblyomma lepidum | 0.26% (1/387) | North Kordofan |
| Babesia caballi | MK288110, AB734386, EU642514 | 97, 98 | Amblyomma variegatum | 1.72% (1/58) | North Kordofan |
| Babesia pecorum | KC249945, KC249944, FJ213577 | 98–99, 100 | Hyalomma impeltatum | 0.33% (2/600) | North Kordofan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Shuaib, Y.A.; Isaa, M.H.; Ezz-Eldin, M.I.-E.; Osman, A.Y.; Yagoub, I.A.; Abdalla, M.A.; Bakiet, A.O.; Mohmed-Noor, S.E.-T.; Schaper, S.; et al. Tick Fauna and Associated Rickettsia, Theileria, and Babesia spp. in Domestic Animals in Sudan (North Kordofan and Kassala States). Microorganisms 2020, 8, 1969. https://doi.org/10.3390/microorganisms8121969
Springer A, Shuaib YA, Isaa MH, Ezz-Eldin MI-E, Osman AY, Yagoub IA, Abdalla MA, Bakiet AO, Mohmed-Noor SE-T, Schaper S, et al. Tick Fauna and Associated Rickettsia, Theileria, and Babesia spp. in Domestic Animals in Sudan (North Kordofan and Kassala States). Microorganisms. 2020; 8(12):1969. https://doi.org/10.3390/microorganisms8121969
Chicago/Turabian StyleSpringer, Andrea, Yassir Adam Shuaib, Makarim Habib Isaa, Malaz Isam-Eldin Ezz-Eldin, Abdinasir Yusuf Osman, Idris Ahmed Yagoub, Mohamed Abdalsalam Abdalla, Amel Omer Bakiet, Saad El-Tiab Mohmed-Noor, Sabine Schaper, and et al. 2020. "Tick Fauna and Associated Rickettsia, Theileria, and Babesia spp. in Domestic Animals in Sudan (North Kordofan and Kassala States)" Microorganisms 8, no. 12: 1969. https://doi.org/10.3390/microorganisms8121969