Three Novel Clostridia Isolates Produce n-Caproate and iso-Butyrate from Lactate: Comparative Genomics of Chain-Elongating Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrichment, Isolation, and Identification of Lactate-Consuming Strains
2.2. Batch Cultivation
2.3. Analytical Techniques
2.4. Gene Prediction and Annotation
2.5. Phylogenetic Analysis and Taxonomic Classification
2.6. Pan-Genome Analysis
2.7. Data Availability
3. Results and Discussion
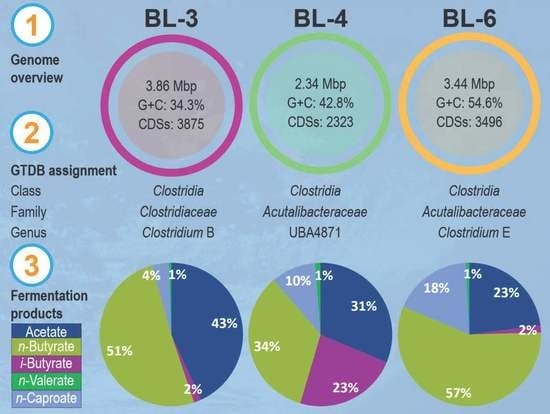
3.1. Isolation and Identification of Lactate-Consuming Strains
3.2. Conversion of Lactate to n-Caproate and iso-Butyrate in Batch Cultivation
3.3. Genomic Heterogeneity of Strains BL-3, BL-4, and BL-6
3.4. Genomic Diversity of the Reported Chain-Elongating Bacterial Strains
3.5. Genetic Basis of Lactate Conversion to n-Caproate and iso-Butyrate
3.5.1. Lactate Oxidation to Acetyl-CoA
3.5.2. Ethanol Oxidation to Acetyl-CoA
3.5.3. n-Butyrate and n-Caproate Formation
3.5.4. Energy Conservation and Hydrogen Formation
3.5.5. iso-Butyrate Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.J.; Plugge, C.M.; Strik, D.P.B.T.B.; Grootscholten, T.I.M.; Buisman, C.J.N.; Hamelers, H.V.M. Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef]
- Zhang, K.; Woodruff, A.P.; Xiong, M.; Zhou, J.; Dhande, Y.K. A synthetic metabolic pathway for production of the platform chemical isobutyric acid. ChemSusChem 2011, 4, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, K.D.; De Smit, S.M.; De Oossanen, S.; Van Moerland, M.J.; Buisman, C.J.N.; Strik, D.P.B.T.B. Methanol-based chain elongation with acetate to n-butyrate and isobutyrate at varying selectivities dependent on pH. ACS Sustain. Chem. Eng. 2020, 8, 8184–8194. [Google Scholar] [CrossRef]
- Scalschi, L.; Vicedo, B.; Camañes, G.; Fernandez-Crespo, E.; Lapeña, L.; González-Bosch, C.; García-Agustín, P. Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Mol. Plant Pathol. 2013, 14, 342–355. [Google Scholar] [CrossRef]
- Urban, C.; Xu, J.; Sträuber, H.; dos Santos, T.R.D.; Mühlenberg, J.; Härtig, C.; Angenet, L.T.; Harnisch, F. Production of drop-in fuel from biomass by combined microbial and electrochemical conversions. Energy Environ. Sci. 2017, 10, 2231–2244. [Google Scholar] [CrossRef]
- Evans, N.P.; Collins, D.A.; Pierson, F.W.; Mahsoub, H.M.; Sriranganathan, N.; Persia, M.E.; Karnezos, T.P.; Sims, M.D.; Dalloul, R.A. Investigation of medium chain fatty acid feed supplementation for reducing Salmonella typhimurium colonization in turkey poults. Foodborne Pathog. Dis. 2017, 14, 531–536. [Google Scholar] [CrossRef]
- Kenealy, W.R.; Cao, Y.; Weimer, P.J. Production of caproic acid by cocultures of ruminal cellulolytic bacteria and Clostridium kluyveri grown on cellulose and ethanol. Appl. Microbiol. Biotechnol. 1995, 44, 507–513. [Google Scholar] [CrossRef]
- Anneken, D.J.; Both, S.; Christoph, R.; Fieg, G.; Steinberner, U.; Westfechtel, A. Fatty Acids. Ullmann’s Encycl. Ind. Chem. 2006, 14, 73–116. [Google Scholar]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- De Leeuw, K.D.; Buisman, C.J.N.; Strik, D.P.B.T.B. Branched medium chain fatty acids: Iso-caproate formation from iso-butyrate broadens the product spectrum for microbial chain elongation. Environ. Sci. Technol. 2019, 53, 7704–7713. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Huang, S.; Strik, D.P.B.T.B.; Buisman, C.J.N. Isobutyrate biosynthesis via methanol chain elongation: Converting organic wastes to platform chemicals. J. Chem. Technol. Biotechnol. 2017, 92, 1370–1379. [Google Scholar] [CrossRef]
- Huang, S.; Kleerebezem, R.; Rabaey, K.; Ganigué, R. Open microbiome dominated by Clostridium and Eubacterium converts methanol into i-butyrate and n-butyrate. Appl. Microbiol. Biotechnol. 2020, 104, 5119–5131. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, H.; Fricke, W.F.; Veith, B.; Brüggemann, H.; Liesegang, H.; Strittmatter, A.; Miethke, M.; Buckel, W.; Hinderberger, J.; Li, F.; et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. USA 2008, 105, 2128–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhou, Y.; Wang, Y.; Wu, T.; Li, X.; Li, D.; Tao, Y. Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnol. Biofuels 2017, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Van Brabant, P. Understanding Bio-Isomerisation during Methanol Fermentation. Master’s Thesis, Ghent University, Ghent, Belgium, 2019. [Google Scholar]
- Lambrecht, J.; Cichocki, N.; Schattenberg, F.; Kleinsteuber, S.; Harms, H.; Müller, S.; Sträuber, H. Key sub-community dynamics of medium-chain carboxylate production. Microb. Cell Fact. 2019, 18, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Kleinsteuber, S.; Centler, F.; Harms, H.; Sträuber, H. Competition between butyrate fermenters and chain-elongating bacteria limits the efficiency of medium-chain carboxylate production. Front. Microbiol. 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Popp, D.; Sträuber, H.; Harms, H.; Kleinsteuber, S. Draft genome sequences of three Clostridia isolates involved in lactate-based chain elongation. Microbiol. Resour. Announc. 2020, 9, e00679-20. [Google Scholar] [CrossRef]
- Müller, N.; Griffin, B.M.; Stingl, U.; Schink, B. Dominant sugar utilizers in sediment of Lake Constance depend on syntrophic cooperation with methanogenic partner organisms. Environ. Microbiol. 2008, 10, 1501–1511. [Google Scholar] [CrossRef] [Green Version]
- Widdel, F.; Kohring, G.W.; Mayer, F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids-III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch. Microbiol. 1983, 134, 286–294. [Google Scholar] [CrossRef]
- Widdel, F.; Bak, F. Gram-Negative Mesophilic Sulfate-Reducing Bacteria. In The Prokaryotes; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.H., Eds.; Springer: Berlin, Germany, 1992; pp. 3352–3378. [Google Scholar]
- Widdel, F.; Pfennig, N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids-I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch. Microbiol. 1981, 129, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Junghare, M.; Müller, N. Fermentation of glycerol by Anaerobium acetethylicum and its potential use in biofuel production. Microb. Biotechnol. 2017, 10, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Sträuber, H.; Schröder, M.; Kleinsteuber, S. Metabolic and microbial community dynamics during the hydrolytic and acidogenic fermentation in a leach-bed process. Energy Sustain. Soc. 2012, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Vallenet, D.; Calteau, A.; Dubois, M.; Amours, P.; Bazin, A.; Beuvin, M.; Burlot, L.; Bussell, X.; Fouteau, S.; Gautreau, G.; et al. MicroScope: An integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res. 2019, 48, D579–D589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Smirnov, S.; Nikolskaya, A.N.; et al. The COG database: An updated vesion includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; Von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Overbeek, R.; Rodriguez, A. FIGfams: Yet another set of protein families. Nucleic Acids Res. 2009, 37, 6643–6654. [Google Scholar] [CrossRef]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L.; et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- Chevenet, F.; Brun, C.; Bañuls, A.L.; Jacq, B.; Christen, R. TreeDyn: Towards dynamic graphics and annotations for analyses of trees. BMC Bioinform. 2006, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Blondel, V.D.; Guillaume, J.L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 10, P10008. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Ha, S.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Vallenet, D.; Calteau, A.; Cruveiller, S.; Gachet, M.; Lajus, A.; Josso, A.; Mercier, J.; Renaux, A.; Rollin, J.; Rouy, Z.; et al. MicroScope in 2017: An expanding and evolving integrated resource for community expertise of microbial genomes. Nucleic Acids Res. 2017, 45, D517–D528. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Ebers, J. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Scarborough, M.J.; Myers, K.S.; Donohue, T.J.; Noguera, D.R. Medium-chain fatty acid synthesis by “Candidatus Weimeria bifida” gen. nov., sp. nov., and “Candidatus Pseudoramibacter fermentans” sp. nov. Appl. Environ. Microbiol. 2020, 86, e02242-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, T.B.; Humphrey, S.B. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl. Environ. Microbiol. 2003, 69, 3874–3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimer, P.J.; Moen, G.N. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, X.; Wang, H.; Wang, Y.; Li, X.; Jin, H.; Rui, J. Complete genome sequence of Ruminococcaceae bacterium CPB6: A newly isolated culture for efficient n-caproic acid production from lactate. J. Biotechnol. 2017, 259, 91–94. [Google Scholar] [CrossRef]
- Jeon, B.S.; Kim, S.; Sang, B.I. Megasphaera hexanoica sp. Nov., a medium-chain carboxylic acid-producing bacterium isolated from a cow rumen. Int. J. Syst. Evol. Microbiol. 2017, 67, 2114–2120. [Google Scholar] [CrossRef]
- Willems, A.; Collins, M.D. Phylogenetic relationships of the genera Acetobacterium and Eubacterium sensu stricto and reclassification of Eubacterium alactolyticum as Pseudoramibacter alactolyticus gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1996, 46, 1083–1087. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Cato, E.P.; Moore, W.E.C. Amended description of Ramibacterium alactolyticum Prevot and Taffanel with proposal of a neotype strain. Int. J. Syst. Bacteriol. 1967, 17, 323–341. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeon, B.S.; Kim, S.; Kim, H.; Um, Y.; Sang, B.I. Caproiciproducens galactitolivorans gen. nov., sp. nov., a bacterium capable of producing caproic acid from galactitol, isolated from a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2015, 65, 4902–4908. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Poehlein, A.; Daniel, R.; Dürre, P. Genome sequence of the caproic acid-producing bacterium Caproiciproducens galactitolivorans BS-1T (JCM 30532). Microbiol. Resour. Announc. 2019, 8, e00346-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genthner, B.R.; Davis, C.L.; Bryant, M.P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl. Environ. Microbiol. 1981, 42, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.J.; McKain, N.; McEwan, N.R.; Miyagawa, E.; Chaudhary, L.C.; King, T.P.; Walker, N.D.; Apajalahti, J.H.A.; Newbold, C.J. Eubacterium pyruvativorans sp. nov., a novel non-saccharolytic anaerobe from the rumen that ferments pyruvate and amino acids, forms caproate and utilizes acetate and propionate. Int. J. Syst. Evol. Microbiol. 2003, 53, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Chaudhary, L.C.; Miyagawa, E.; McKain, N.; Walker, N.D. Metabolic properties of Eubacterium pyruvativorans, a ruminal ‘hyper-ammonia-producing’ anaerobe with metabolic properties analogous to those of Clostridium kluyveri. Microbiology 2004, 150, 2921–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, C.D.; Li, C.H.; Li, J.G.; Chen, Q.; Li, Y.Z. Clostridium luticellarii sp. nov., isolated from a mud cellar used for producing strong aromatic liquors. Int. J. Syst. Evol. Microbiol. 2015, 65, 4730–4733. [Google Scholar] [CrossRef]
- Poehlein, A.; Bremekamp, R.; Lutz, V.T.; Schulz, L.M.; Daniel, R. Draft genome sequence of the butanoic acid-producing bacterium Clostridium luticellarii DSM 29923, used for strong aromatic Chinese liquor production. Genome Announc. 2018, 6, e00377-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, R.; Silva, F.J.; Pereto, J.; Moya, A. Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev. 2004, 68, 518–537. [Google Scholar] [CrossRef] [Green Version]
- Agler, M.T.; Spirito, C.M.; Usack, J.G.; Werner, J.J.; Angenent, L.T. Chain elongation with reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5, 8189. [Google Scholar] [CrossRef]
- Kucek, L.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. Chain elongation of acetate and ethanol in an upflow anaerobic filter for high rate MCFA production. Bioresour. Technol. 2013, 135, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. The coenzyme A transphorase system in Clostridium kluyveri. J. Biol. Chem. 1953, 203, 501–512. [Google Scholar] [PubMed]
- Stadtman, E.R. Functional group of coenzyme A and its metabolic relations, especially in the fatty acid cycle. Discuss. Fed. Proc. 1953, 12, 692–693. [Google Scholar]
- Scarborough, M.J.; Hamilton, J.J.; Erb, E.A.; Donohue, T.J.; Noguera, D.R. Diagnosing and predicting mixed-culture fermentations with unicellular and guild-based metabolic models. mSystems 2020, 5, e00755-20. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef] [Green Version]
- Buckel, W.; Thauer, R.K. Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem. Rev. 2018, 118, 3862–3886. [Google Scholar] [CrossRef] [Green Version]
- Buckel, W.; Thauer, R.K. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 94–113. [Google Scholar] [CrossRef] [Green Version]
- Hedderich, R.; Forzi, L. Energy-converting [NiFe] hydrogenases: More than just H2 activation. J. Mol. Microbiol. Biotechnol. 2006, 10, 92–104. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Weghoff, M.C.; Bertsch, J.; Müller, V. A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol. 2015, 17, 670–677. [Google Scholar] [CrossRef]
- Tholozan, J.L.; Samain, E.; Grivet, J.P. Isomerization between n-butyrate and isobutyrate in enrichment cultures. FEMS Microbiol. Lett. 1988, 53, 187–191. [Google Scholar] [CrossRef]
- Matthies, C.; Schink, B. Reciprocal isomerization of butyrate and isobutyrate by the strictly anaerobic bacterium strain WoG13 and methanogenic isobutyrate degradation by a defined triculture. Appl. Environ. Microbiol. 1992, 58, 1435–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cracan, V.; Padovani, D.; Banerjee, R. IcmF is a fusion between the radical B12 enzyme isobutyryl-CoA mutase and its G-protein chaperone. J. Biol. Chem. 2010, 285, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, H.A. Coenzyme Bl2-Dependent mutases causing carbon chain rearrangements. Enzymes 1972, 6, 509–537. [Google Scholar] [CrossRef]
- Allison, M.J. Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl. Environ. Microbiol. 1978, 35, 872–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieb, M.; Schink, B. Anaerobic degradation of isobutyrate by methanogenic enrichment cultures and by a Desulfococcus multivorans strain. Arch. Microbiol. 1989, 151, 126–132. [Google Scholar] [CrossRef]
- Marshall, V.D.; Sokatch, J.R. Regulation of valine catabolism in Pseudomonas putida. J. Bacteriol. 1972, 110, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Gollop, N.; Damri, B.; Barak, Z.; Chipman, D.M. Kinetics and mechanism of acetohydroxy acid synthase isozyme III from Escherichia coli. Biochemistry 1989, 28, 6310–6317. [Google Scholar] [CrossRef]








| Strain | GTDB Taxonomy | Isolation Source | Genome Size (bp) | GC Content (%) | No. of Predicted CDSs | Reference |
|---|---|---|---|---|---|---|
| BL-3 | Clostridium_B | Anaerobic bioreactor | 3,855,691 | 34.32 | 3875 | [19] |
| BL-4 | Acutalibacteraceae UBA4871 | Anaerobic bioreactor | 2,335,857 | 42.75 | 2323 | [19] |
| BL-6 | Clostridium_E sp002397665 | Anaerobic bioreactor | 3,435,529 | 54.63 | 3496 | [19] |
| Megasphaera elsdenii 14-14 | Megasphaera elsdenii | Human gut | 2,504,349 | 52.75 | 2359 | [47,48] |
| Ruminococcaceae bacterium CPB6 | Acutalibacteraceae UBA4871 sp002119605 | Sludge of a caproate-producing reactor | 2,069,994 | 50.58 | 2116 | [15,49] |
| Megasphaera hexanoica MH | Caecibacter massiliensis | Cow rumen | 2,877,851 | 49.00 | 2799 | [50] |
| Pseudoramibacter alactolyticus ATCC 23263 | Pseudoramibacter alactolyticus | Human oral cavity | 2,366,982 | 51.63 | 2327 | [51,52] |
| Candidatus Pseudoramibacter fermentans a | Pseudoramibacter sp002396065 | Anaerobic bioreactor | 2,288,358 | 50.15 | 2209 | [46] |
| Clostridium kluyveri DSM 555 | Clostridium_B kluyveri | Canal mud | 4,023,800 | 32.02 | 4371 | [14] |
| Caproiciproducens galactitolivorans BS-1 | Acutalibacteraceae MS4 | Anaerobic digester sludge | 2,578,839 | 48.10 | 2539 | [53,54] |
| Eubacterium limosum KIST612 | Eubacterium limosum | Sheep rumen | 4,740,532 | 46.86 | 4605 | [51,55] |
| Eubacterium pyruvativorans i6 | Eubacterium_A pyruvativorans | Sheep rumen | 2,164,212 | 54.84 | 1954 | [56,57] |
| Candidatus Weimeria bifida a | Lachnospiraceae UBA2727 | Anaerobic bioreactor | 2,395,883 | 45.93 | 2477 | [46] |
| Clostridium luticellarii DSM 29923 | Clostridium_B luticellarii | Mud cellar | 3,771,178 | 34.97 | 3874 | [58,59] |
| Predicted Function | No. | Enzyme Abbreviation | EC Number | Enzyme |
|---|---|---|---|---|
| Acetyl-CoA formation | 1 | LacR | 5.1.2.1 | Lactate racemase |
| 2 | LacP | 2.A.14 | Lactate permease | |
| 3 | LDH | 1.1.1.27 | Lactate dehydrogenase | |
| 4 | PFOR | 1.2.7.1 | Pyruvate ferredoxin oxidoreductase | |
| 5 | ADH | 1.1.1.1 | Alcohol dehydrogenase | |
| 6 | ADA | 1.2.1.10 | Acetaldehyde dehydrogenase | |
| Reverse β-oxidation | 7 | ACAT | 2.3.1.9, 2.3.1.16 | Acetyl-CoA acetyltransferase |
| 8 | HAD | 1.1.1.157, 1.1.1.35 | 3-Hydroxyacyl-CoA dehydrogenase | |
| 9 | ECH | 4.2.1.150, 4.2.1.55 | Enoyl-CoA hydratase | |
| 10 | BCD | 1.3.8.1 | Butyryl-CoA dehydrogenase | |
| 11 | EtfAB | Electron transfer flavoprotein A,B | ||
| 12 | CoAT | 2.8.3.- | Butyryl-CoA:acetate CoA-transferase | |
| 13 | ACT | 3.1.2.20 | Acyl-CoA thioesterase | |
| Energy conservation | 14 | RnfABCDEG | 7.1.1.1 | Energy-converting NADH:ferredoxin oxidoreductase |
| 15 | EchABCDEF | Energy-converting hydrogenase | ||
| H2 formation | 16 | H2ase | 1.12.7.2 | Hydrogen:ferredoxin oxidoreductase |
| Butyrate formation | 17 | PTB | 2.3.1.19 | Phosphate butyryltransferase |
| 18 | BUK | 2.7.2.7 | Butyrate kinase | |
| Others | 19 | BM | 5.4.99.13 | Butyryl-CoA:isobutyryl-CoA mutase |
| 20 | ACOCT | 2.8.3.19 | Acetyl-CoA:oxalate CoA-transferase | |
| 21 | HadABC | 4.2.1.157 | (R)-2-hydroxyisocaproyl-CoA dehydratase | |
| 22 | CarC | 1.3.1.108 | Caffeyl-CoA reductase-Etf complex subunit CarC | |
| 23 | HypCDEF | Hydrogenase maturation factor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Popp, D.; Müller, N.; Sträuber, H.; Harms, H.; Kleinsteuber, S. Three Novel Clostridia Isolates Produce n-Caproate and iso-Butyrate from Lactate: Comparative Genomics of Chain-Elongating Bacteria. Microorganisms 2020, 8, 1970. https://doi.org/10.3390/microorganisms8121970
Liu B, Popp D, Müller N, Sträuber H, Harms H, Kleinsteuber S. Three Novel Clostridia Isolates Produce n-Caproate and iso-Butyrate from Lactate: Comparative Genomics of Chain-Elongating Bacteria. Microorganisms. 2020; 8(12):1970. https://doi.org/10.3390/microorganisms8121970
Chicago/Turabian StyleLiu, Bin, Denny Popp, Nicolai Müller, Heike Sträuber, Hauke Harms, and Sabine Kleinsteuber. 2020. "Three Novel Clostridia Isolates Produce n-Caproate and iso-Butyrate from Lactate: Comparative Genomics of Chain-Elongating Bacteria" Microorganisms 8, no. 12: 1970. https://doi.org/10.3390/microorganisms8121970