Structural Synergy of NanoAl2O3/NanoAl Composites with High Thermomechanical Properties and Ductility
Abstract
:1. Introduction
2. Experiments
3. Results
3.1. Microstructure of NanoAl2O3/NanoAl Composites
3.2. Mechanical Properties of NanoAl2O3/nanoAl Composites at 25 and 500 °C
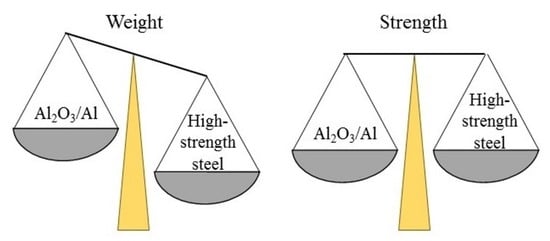
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koli, D.K.; Agnihotri, G.; Purohit, R. A Review on properties, behaviour and processing methods for Al-nano Al2O3 composites. Proc. Mater. Sci. 2014, 6, 567–589. [Google Scholar] [CrossRef]
- Rezayat, M.; Akbarzadeh, A.; Owhadi, A. Production of high strength Al–Al2O3 composite by accumulative roll bonding. Comp. Part A 2012, 43, 261–267. [Google Scholar] [CrossRef]
- Garbiec, D.; Jurczyk, M.; Levintant-Zayonts, N.; Mościcki, T. Properties of Al-Al2O3 composites synthesized by spark plasma sintering method. Acrh. Civil Mech. Eng. 2015, 15, 933–939. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Włodarczyk, A.; Adamiak, M. The structure and properties of PM composite materials based on EN AW-2124 aluminum alloy reinforced with the BN or Al2O3 ceramic particles. J. Mater. Process. Technol. 2006, 175, 186–191. [Google Scholar] [CrossRef]
- Oh, K.H.; Han, K.S. Short-fiber/particle hybrid reinforcement: Effects on fracture toughness and fatigue crack growth of metal matrix composites. Compos. Sci. Technol. 2007, 67, 1719–1726. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Torabi Parizi, M.; Sajjadi, S.A.; Ebrahimi, G.R.; Chaichi, A. Microstructure, mechanical analysis and optimal selection of 7075 aluminum alloy based composite reinforced with alumina nanoparticles. Mater. Chem. Phys. 2016, 178, 119–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, G.; Tan, Z.; Zhao, H.; Xu, Y.; Xiong, D.; Li, Z. Towards the strength-ductility synergy of Al2O3/Al composite through the design of roughened interface. Comp. Part B 2021, 224, 109251. [Google Scholar] [CrossRef]
- Yu, S.; Li, W.; He, Z. Study on tensile strengths of Al2O3 short fiber reinforced Zn–Al alloy composites at elevated temperatures. J. Antimicrob. Chemother. 2007, 431, L8–L11. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Chao, C.-G. Deformation and fracture of Al2O3/Al–Zn–Mg–Cu metal matrix composites at room and elevated temperatures. Mater. Sci. Eng. A 2000, 282, 193–202. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, F.; Zhao, Q.; Zha, M.; Jiang, Q. Superior high creep resistance of in situ nano-sized TiCx/Al-Cu-Mg composite. Sci. Rep. 2017, 7, 4540. [Google Scholar] [CrossRef]
- Zan, Y.-N.; Zhou, Y.-T.; Li, X.-N.; Ma, G.-N.; Liu, Z.-Y.; Wang, Q.-Z.; Wang, D.; Xiao, B.-L.; Ma, Z.-Y. Enhancing high-temperature strength and thermal stability of Al2O3/Al composites by high-temperature pre-treatment of ultrafine Al powders. Acta Metall. Sin. Engl. Lett. 2020, 33, 913–921. [Google Scholar] [CrossRef]
- Balog, M.; Krizik, P.; Nosko, M.; Hajovska, Z.; Victoria Castro Riglos, M.; Rajner, W.; Liu, D.-S.; Simancik, F. Forged HITEMAL: Al-based MMCs strengthened with nanometric thick Al2O3 skeleton. Mater. Sci. Eng. A 2014, 613, 82–90. [Google Scholar] [CrossRef]
- Corthay, S.; Kutzhanov, M.K.; Matveev, A.T.; Bondarev, A.V.; Leybo, D.V.; Shtansky, D.V. Nanopowder derived Al/h-BN composites with high strength and ductility. J. Alloys Compd. 2022, 912, 165199. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Sanaty-Zadeh, A.; Rohatgi, P.K. Comparison between current models for the strength of particulate-reinforced metal matrix nanocomposites with emphasis on consideration of Hall–Petch effect. Mater. Sci. Eng. A 2012, 531, 112–118. [Google Scholar] [CrossRef]
- Chen, X.-H.; Yan, H. Fabrication of nanosized Al2O3 reinforced aluminum matrix composites by subtype multifrequency ultrasonic vibration. J. Mater. Res. 2015, 30, 2197–2209. [Google Scholar] [CrossRef]
- Tellkamp, V.L.; Lavernia, E.J.; Melmed, A. Mechanical behavior and microstructure of a thermally stable bulk nanostructured Al alloy. Metall. Mat. Trans. A 2001, 32, 2335–2343. [Google Scholar] [CrossRef]
- Han, B.O.; Lavernia, E.J.; Lee, Z.; Nutt, S.; Witkin, D. Deformation behavior of bimodal nanostructured 5083 Al alloys. Metall. Mat. Trans. A 2005, 36, 957–965. [Google Scholar] [CrossRef]
- Ye, J.; Han, B.Q.; Lee, Z.; Ahn, B.; Nutt, S.R.; Schoenung, J.M. A tri-modal aluminum based composite with super-high strength. Scr. Mater. 2005, 53, 481–486. [Google Scholar] [CrossRef]
- Taya, M.; Lulay, K.E.; Lloyd, D.J. Strengthening of a particulate metal matrix composite by quenching. Acta Metall. Mater. 1991, 39, 73–87. [Google Scholar] [CrossRef]
- Casati, R.; Fabrizi, A.; Timelli, G.; Tuissi, A.; Vedani, M. Microstructural and mechanical properties of Al-based composites reinforced with in-situ and ex-situ Al2O3 nanoparticles. Adv. Eng. Mater. 2016, 18, 550–558. [Google Scholar] [CrossRef]
- Lubyanskiy, Y.V.; Bondarev, A.D.; Soshnikov, I.P.; Bert, N.A.; Zolotarev, V.V.; Kirilenko, D.A.; Kotlyar, K.P.; Pikhtin, N.A.; Tarasov, I.S. Oxygen nitrogen mixture effect on aluminum nitride synthesis by reactive ion plasma deposition. Semiconductors 2018, 52, 184–188. [Google Scholar] [CrossRef]
- Kutzhanov, M.K.; Matveev, A.T.; Bondarev, A.V.; Polcar, T.; Duchoň, J.; Shtansky, D.V. Al-based composites reinforced with ceramic particles formed by in situ reactions between Al and amorphous SiNxOy. Mater. Sci. Eng. A 2022, 842, 143105. [Google Scholar] [CrossRef]
- Casati, R.; Fabrizi, A.; Tuissi, A.; Xia, K.; Vedani, M. ECAP consolidation of Al matrix composites reinforced with in-situ γ-Al2O3 nanoparticles. Mater. Sci. Eng. A 2015, 648, 113–122. [Google Scholar] [CrossRef]
- Esmaeily, A.S.; Mills, S.; Coey, J.M.D. Exceptional room-temperature plasticity in amorphous alumina nanotubes fabricated by magnetic hard anodization. Nanoscale 2017, 9, 5205–5211. [Google Scholar] [CrossRef]
- Balog, M.; Hu, T.; Krizik, P.; Riglos, M.V.C.; Saller, B.D.; Yang, H.; Schoenung, J.M.; Lavernia, E.J. On the thermal stability of ultrafine-grained Al stabilized by in-situ amorphous Al2O3 network. Mater. Sci. Eng. A 2015, 648, 61–71. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Liu, E.; Li, Q.; He, C.; Shi, C.; Zhao, N. Synergistic strengthening effect of alumina anchored graphene nanosheets hybrid structure in aluminum matrix composites. Fuller. Nanotub. Carbon Nanostructures 2019, 27, 640–649. [Google Scholar] [CrossRef]
- Sadeghi, B.; Cavaliere, P.; Balog, M.; Pruncu, C.I.; Shabani, A. Microstructure dependent dislocation density evolution in micro-macro rolled Al2O3/Al laminated composite. Mater. Sci. Eng. A 2022, 830, 142317. [Google Scholar] [CrossRef]
- Singh, T.; Tiwari, S.K.; Shukla, D.K. Effects of Al2O3 nanoparticles volume fractions on microstructural and mechanical characteristics of friction stir welded nanocomposites. Nanocomposites 2020, 6, 76–84. [Google Scholar] [CrossRef]
- Leparoux, M.; Kollo, L.; Kwon, H.; Kallip, K.; Babu, N.K.; AlOgab, K.; Talari, M.K. Solid state processing of aluminum matrix composites reinforced with nanoparticulate materials. Adv. Eng. Mater. 2018, 20, 1800401. [Google Scholar] [CrossRef]
- Krizik, P.; Balog, M.; Nagy, S. Small punch testing of heat resistant ultrafine-grained Al composites stabilized by nano-metric Al2O3 (HITEMAL©) in a broad temperature range. J. Alloys Compd. 2021, 887, 161332. [Google Scholar] [CrossRef]
- Zaiemyekeh, Z.; Liaghat, G.H.; Ahmadi, H.; Khan, M.K.; Razmkhah, O. Effect of strain rate on deformation behavior of aluminum matrix composites with Al2O3 nanoparticles. Mater. Sci. Eng. A 2019, 753, 276–284. [Google Scholar] [CrossRef]
- Vogel, T.; Ma, S.; Liu, Y.; Guo, Q.; Zhang, D. Impact of alumina content and morphology on the mechanical properties of bulk nanolaminated Al2O3-Al composites. Compos. Commun. 2020, 22, 100462. [Google Scholar] [CrossRef]
- Reddy, M.P.; Ubaid, F.; Shakoor, R.A.; Parande, G.; Manakari, V.; Mohamed, A.M.A.; Gupta, M. Effect of reinforcement concentration on the properties of hot extruded Al-Al2O3 composites synthesized through microwave sintering process. Mater. Sci. Eng. A 2017, 696, 60–69. [Google Scholar] [CrossRef]
- Hilditch, T.B.; de Souza, T.; Hodgson, P.D. Properties and automotive applications of advanced high-strength steels (AHSS). In Welding and Joining of Advanced High Strength Steels (AHSS); Shome, M., Tumuluru, M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 9–28. [Google Scholar] [CrossRef]
- Balog, M.; Krizik, P.; Yan, M.; Simancik, F.; Schaffer, G.B.; Qian, M. SAP-like ultrafine-grained Al composites dispersion strengthened with nanometric AlN. Mater. Sci. Eng. A 2013, 588, 181–187. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Yang, Y.; Gupta, N.; Yang, C.; Zhao, Y.; Hu, Z. High-temperature mechanical properties of aluminum alloy matrix composites reinforced with Zr and Ni trialumnides synthesized by in situ reaction. Metall. Mater. Trans. A 2020, 51, 214–225. [Google Scholar] [CrossRef]
- Corthay, S.; Firestein, K.L.; Kvashnin, D.G.; Kutzhanov, M.K.; Matveev, A.T.; Kovalskii, A.M.; Leybo, D.V.; Golberg, D.V.; Shtansky, D.V. Elevated-temperature high-strength h-BN-doped Al2014 and Al7075 composites: Experimental and theoretical insights. Mater. Sci. Eng. A 2021, 809, 140969. [Google Scholar] [CrossRef]
- Summers, P.T.; Chen, Y.; Rippe, C.M.; Allen, B.; Mouritz, A.P.; Case, S.W.; Lattimer, B.Y. Overview of aluminum alloy mechanical properties during and after fires. Fire Sci. Rev. 2015, 4, 3. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Murashkin, M.Y.; Sabirov, I. A nanostructural design to produce high-strength Al alloys with enhanced electrical conductivity. Scr. Mater. 2014, 76, 13–16. [Google Scholar] [CrossRef]
- Senkova, S.V.; Senkov, O.N.; Miracle, D.B. Cryogenic and elevated temperature strengths of an Al−Zn−Mg−Cu alloy modified with Sc and Zr, Metall. Mater. Trans. A 2006, 37, 3569–3575. [Google Scholar] [CrossRef]
- Inoue, A.; Kimura, H. High-strength aluminum alloys containing nanoquasicrystalline particles. Mater. Sci. Eng. A 2000, 286, 1–10. [Google Scholar] [CrossRef]
- Pickens, J.R. High-strength aluminum P/M alloys. In ASM Handbook Committee; ASM: Almere, The Netherlands, 1990; Volume 2, pp. 200–215. [Google Scholar]
- Ma, X.; Zhao, Y.F.; Tian, W.J.; Qian, Z.; Chen, H.W.; Wu, Y.Y.; Liu, X.F. A novel Al matrix composite reinforced by nano-AlNp network. Sci. Rep. 2016, 6, 34919. [Google Scholar] [CrossRef] [PubMed]
- Firestein, K.L.; Corthay, S.; Steinman, A.E.; Matveev, A.T.; Kovalskii, A.M.; Sukhorukova, I.V.; Golberg, D.; Shtansky, D.V. High-strength aluminum-based composites reinforced with BN, AlB2 and AlN particles fabricated via reactive spark plasma sintering of Al-BN powder mixtures. Mater. Sci. Eng. A 2017, 681, 1–9. [Google Scholar] [CrossRef]
- Kutzhanov, M.K.; Matveev, A.T.; Kvashnin, D.G.; Corthay, S.; Jalolov, F.N.; Kvashnin, A.G.; Konopatsky, A.S.; Leybo, D.V.; Bondarev, A.V.; Arkhipova, N.A.; et al. Al/SiC composites with enhanced thermomechanical properties obtained from microwave plasma-treated nanopowders. Mater. Sci. Eng. A 2021, 824, 141817. [Google Scholar] [CrossRef]
- Baek, M.-S.; Euh, K.; Lee, K.-A. Microstructure, tensile and fatigue properties of high strength Al 7075 alloy manufactured via twin-roll strip casting. J. Mater. Res. Technol. 2020, 9, 9941–9950. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Progress Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Liao, X.Z.; Wu, X.L. Deformation twinning in nanocrystalline materials. Prog. Mater. Sci. 2012, 57, 1–62. [Google Scholar] [CrossRef]
- Kou, Z.; Yang, Y.; Yang, L.; Zhang, W.; Huang, B.; Luo, X. Deformation twinning in response to cracking in Al: An in situ TEM and molecular dynamics study. Scripta Mater. 2018, 145, 28–32. [Google Scholar] [CrossRef]
- Goswami, R.; Pande, C.S.; Bernstein, N.; Johannes, M.D.; Baker, C.; Villalobos, G. A high degree of enhancement of strength of sputter deposited Al/Al2O3 multilayers upon post annealing. Acta Mater. 2015, 95, 378–385. [Google Scholar] [CrossRef]












| Sample | Hardness | Compressive Properties | Tensile Properties | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (HV5) | RT | 500 °C | RT | 500 °C | |||||
| UCS (MPa) | El (%) | UCS (MPa) | El (%) | UTS (MPa) | El (%) | UTS (MPa) | El (%) | ||
| Al-0 | 73 ± 8 | 178 ± 8 | 19.0 ± 1.1 | 100 ± 5 | 12.2 ± 1 | 194 ± 5 | 24 ± 0.8 | 153 ± 10 | 18.4 ± 1 |
| Al-1 | 100 ± 9 | 434 ± 9 | 11.5 ± 1.1 | 272 ± 8 | 9.6 ± 1 | 389 ± 7 | 12.3 ± 0.2 | 253 ± 3 | 14.9 ± 0.2 |
| Al-2 | 112 ± 5 | 466 ± 6 | 25.1 ± 1.0 | 366 ± 5 | 22.2 ± 2 | 396 ± 15 | 15.9 ± 1.6 | 256 ± 8 | 14.5 ± 0.8 |
| Al-3 | 114 ± 5 | 489 ± 10 | 17.8 ± 1.8 | 344 ± 7 | 18.2 ± 0.8 | 512 ± 20 | 17.9 ± 2 | 280 ± 11 | 15.0 ± 0.7 |
| Al-4 | 122 ± 8 | 432 ± 7 | 9.1 ± 0.7 | 324 ± 8 | 20.8 ± 1.1 | 362 ± 10 | 12.1 ± 1 | 252 ± 11 | 15.9 ± 0.4 |
| Al-5 | 124 ± 4 | 442 ± 10 | 14.1 ± 1.2 | 295 ± 2 | 18 ± 0.5 | 360 ± 15 | 16.8 ± 1.3 | 245 ± 9 | 17.2 ± 0.6 |
| Al-10 | 133 ± 7 | 526 ± 11 | 16.7 ± 1.9 | 315 ± 12 | 12.5 ± 0.8 | 356 ± 7 | 11.9 ± 1.1 | 206 ± 3 | 13.2 ± 0.1 |
| Al-20 | 154 ± 10 | 518 ± 8 | 4.8 ± 1 | 299 ± 10 | 4.3 ± 0.4 | 160 ± 5 | 5.2 ± 0.9 | 93 ± 3 | 7.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutzhanov, M.K.; Matveev, A.T.; Bondarev, A.V.; Shchetinin, I.V.; Konopatsky, A.S.; Shtansky, D.V. Structural Synergy of NanoAl2O3/NanoAl Composites with High Thermomechanical Properties and Ductility. Metals 2023, 13, 1696. https://doi.org/10.3390/met13101696
Kutzhanov MK, Matveev AT, Bondarev AV, Shchetinin IV, Konopatsky AS, Shtansky DV. Structural Synergy of NanoAl2O3/NanoAl Composites with High Thermomechanical Properties and Ductility. Metals. 2023; 13(10):1696. https://doi.org/10.3390/met13101696
Chicago/Turabian StyleKutzhanov, Magzhan K., Andrei T. Matveev, Andrey V. Bondarev, Igor V. Shchetinin, Anton S. Konopatsky, and Dmitry V. Shtansky. 2023. "Structural Synergy of NanoAl2O3/NanoAl Composites with High Thermomechanical Properties and Ductility" Metals 13, no. 10: 1696. https://doi.org/10.3390/met13101696