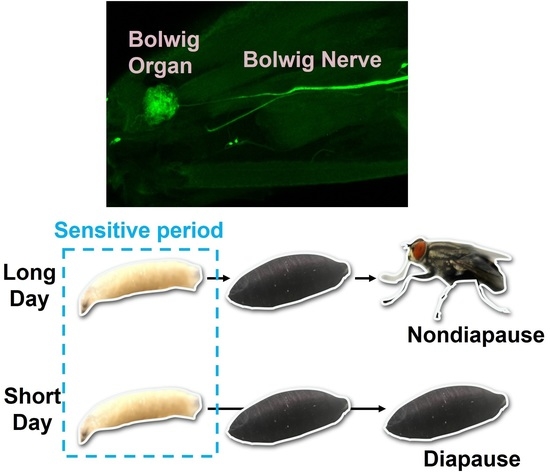
Bolwig Organ and Its Role in the Photoperiodic Response of Sarcophaga similis Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Forward-Fill and Backfill Staining
2.3. Immunohistochemistry
2.4. Microscopy
2.5. Assessment of Photoperiodism
2.6. BO Ablation
2.7. Statistical Testing
3. Results
3.1. Internal Structure of the Anterior End of Wandering Larvae
3.2. Bolwig Organ in S. similis Larvae
3.3. Terminals of the Bolwig-Organ Neurons in the Brain
3.4. Effects of BO Ablation on Photoperiodic Response
4. Discussion
4.1. Bolwig Organ and Its Neuronal Terminals in the Brain of S. similis
4.2. Roles of the BO in Photoperiodism
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford Univ. Press: New York, NY, USA, 1986. [Google Scholar]
- Saunders, D.S. Insect Clocks, 3rd ed.; Elsevier: Amsterdam, The Netherland, 2002. [Google Scholar]
- Saunders, D.S. Insect photoperiodism: Bünning’s hypothesis, the history and development of an idea. Eur. J. Entomol. 2021, 118, 1–13. [Google Scholar] [CrossRef]
- Bünning, E. Die endogene Tagesrhythmik als Grundlage der Photoperiodischen Reaktion. Ber. Dtsch. Bot. Ges. 1936, 54, 590–607. [Google Scholar]
- Saunders, D.S. Dormancy, Diapause, and the role of the circadian system in insect photoperiodism. Annu. Rev. Entomol. 2020, 65, 373–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, D.S. External coincidence and the photoinducible phase in the Sarcophaga photoperiodic clock. J. Comp. Physiol. 1979, 132, 179–189. [Google Scholar] [CrossRef]
- Goto, S.G.; Numata, H. Possible involvement of distinct photoreceptors in the photoperiodic induction of diapause in the flesh fly Sarcophaga similis. J. Insect Physiol. 2009, 55, 401–407. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Minis, D.H. The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am. Nat. 1964, 98, 261–294. [Google Scholar] [CrossRef]
- Meuti, M.E.; Denlinger, D.L. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Boil. 2013, 53, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.G. Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl. Entomol. Zool. 2022, 57, 193–212. [Google Scholar] [CrossRef]
- Tanaka, M.; Tachibana, S.I.; Numata, H. Sensitive stages for photoperiodic induction of pupal diapause in the flesh fly Sarcophaga similis. Appl. Entomol. Zool. 2008, 43, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Raji, J.I.; Potter, C.I. The number of neurons in Drosophila and mosquito brains. PLoS ONE 2021, 16, e0250381. [Google Scholar] [CrossRef]
- Malpel, S.; Klarsfeld, A.; Rouyer, F. Circadian synchronization and rhythmicity in larval photoperception-defective mutants of Drosophila. J. Biol. Rhythms 2004, 19, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.O.; Desplan, C.; Blau, J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron 2005, 45, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolwig, N. Senses and sense organs of the anterior end of the house fly larvae. Vidensk. Medd. Fra Dan. Nat. Foren. Kbh. 1946, 109, 80–212. [Google Scholar]
- Melzer, v.R.R.; Paulus, H. Evolutionary pathways to the larval eyes of insects-higher dipteran stemmata and the evolutionary development of Bolwig’s organ. Z. Zool. Syst. Evol. 1989, 27, 200–245. [Google Scholar] [CrossRef]
- Hinnemann, A.; Niederegger, S.; Hanslik, U.; Heinzel, H.-G.; Spieß, R. See the light: Electrophysiological characterization of the Bolwig organ’s light response of Calliphora vicina 3rd instar larvae. J. Insect Physiol. 2010, 56, 1651–1658. [Google Scholar] [CrossRef]
- Schmucker, D.; Taubert, H.; Jäckle, H. Formation of the Drosophila larval photoreceptor organ and its neuronal differentiation require continuous Krüppel gene activity. Neuron 1992, 9, 1025–1039. [Google Scholar] [CrossRef]
- Malpel, S.; Klarsfeld, A.; Rouyer, F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 2002, 129, 1443–1453. [Google Scholar] [CrossRef]
- Sprecher, S.G.; Pichaud, F.; Desplan, C. Adult and larval photoreceptors use different mechanisms to specify the same rhodopsin fates. Genes Dev. 2007, 21, 2182–2195. [Google Scholar] [CrossRef] [Green Version]
- Keene, A.C.; Mazzoni, E.O.; Zhen, J.; Younger, M.E.; Yamaguchi, S.; Blau, J.; Desplan, C.; Sprecher, S.G. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J. Neurosci. 2011, 31, 6527–6534. [Google Scholar] [CrossRef] [Green Version]
- Sprecher, S.G.; Desplan, C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature 2008, 454, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, E.; Huber, A.; Henrich, S.; Chadwell, L.V.; Chou, W.-H.; Paulsen, R.; Britt, S.G. Blue- and green-absorbing visual pigments of Drosophila: Ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 1999, 19, 10716–10726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helfrich-Förster, C.; Edwards, T.; Yasuyama, K.; Wisotzki, B.; Schneuwly, S.; Stanewsky, R.; Meinertzhagen, I.A.; Hofbauer, A. The extraretinal eyelet of Drosophila: Development, ultrastructure, and putative circadian function. J. Neurosci. 2002, 22, 9255–9266. [Google Scholar] [CrossRef] [PubMed]
- Farca-Luna, A.J.; Sprecher, S.G. Plasticity in the Drosophila larval visual system. Front. Cell. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Martinez, I.; Soler-Cruz, M.D.; Benitez-Rodriguez, R.; Perez-Jimenez, J.M.; Diaz-Lopez, M. Postembryonic development of Wohlfahrtia magnifica (Schiner, 1862) (Diptera: Sarcophagidae). J. Parasitol. 1989, 75, 531–539. [Google Scholar] [CrossRef]
- Yasuyama, K.; Meinertzhagen, I. A Extraretinal photoreceptors at the compound eye’s posterior margin in Drosophila melanogaster. J. Comp. Neurol. 1999, 412, 193–202. [Google Scholar] [CrossRef]
- Yasuyama, K.; Okada, Y.; Hamanaka, Y.; Shiga, S. Synaptic connections between eyelet photoreceptors and pigment dispersing factor-immunoreactive neurons of the blowfly Protophormia terraenovae. J. Comp. Neurol. 2006, 494, 331–344. [Google Scholar] [CrossRef]
- Schoofs, A.; Niederegger, S.; Spieß, R. From behavior to fictive feeding: Anatomy, innervation and activation pattern of pharyngeal muscles of Calliphora vicina 3rd instar larvae. J. Insect Physiol. 2009, 55, 218–230. [Google Scholar] [CrossRef]
- Meinertzhagen, I.A. Development of the compound eye and optic lobe of insects. In Developmental Neurobiology of Arthropods; Young, D., Ed.; Cambridge University Press: London, UK, 1973; pp. 51–104. [Google Scholar]
- Suzuki, T.; Saigo, K. Transcriptional regulation of atonal required for Drosophila larval eye development by concerted action of Eyes absent, Sine oculis and Hedgehog signaling independent of Fused kinase and Cubitus interruptus. Development 2000, 127, 1531–1540. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, L.; Friedrich, M.; Pignoni, F. Distinct regulation of atonal in a visual organ of Drosophila: Organ-specific enhancer and lack of autoregulation in the larval eye. Dev. Biol. 2017, 421, 67–76. [Google Scholar] [CrossRef]
- Steller, H.; Fischbach, K.-F.; Rubin, G.M. disconnected: A locus required for neuronal pathway formation in the visual system of Drosophila. Cell 1987, 50, 1139–1153. [Google Scholar] [CrossRef]
- Kaneko, M.; Helfrich-Förster, C.; Hall, J.C. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: Newly identified pacemaker candidates and novel features of clock gene product cycling. J. Neurosci. 1997, 17, 6745–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Shiga, S.; Goto, S.G. Distribution of PERIOD-immunoreactive neurons and temporal change of the immunoreactivity under long-day and short-day conditions in the larval brain of the flesh fly Sarcophaga similis. Chronobiol. Int. 2017, 34, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Helfrich-Förster, C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1995, 92, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Shiga, S.; Numata, H. Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J. Exp. Biol. 2009, 212, 867–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Q.; Xiang, Y.; Yan, Z.; Han, C.; Jan, L.Y.; Jan, Y.N. Light-induced structural and functional plasticity in Drosophila larval visual system. Science 2011, 333, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarsfeld, A.; Malpel, S.; Michard-Vanhée, C.; Picot, M.; Chélot, E.; Rouyer, F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J. Neurosci. 2004, 24, 1468–1477. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Yuan, Q.; Vogt, N.; Looger, L.L.; Jan, L.Y.; Jan, Y.N.l. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010, 468, 921–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, N.; Schleicher, E.; Kacprzak, S.; Bouly, J.-P.; Picot, M.; Wu, W.; Berndt, A.; Wolf, E.; Bittl, R.; Ahmad, M. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008, 6, e160. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.G.; Numata, H. Alteration of the pupal diapause program and regulation of larval duration by photoperiod in the flesh fly Sarcophaga similis Meade (Diptera: Sarcophagidae). App. Entomol. Zool. 2009, 44, 603–606. [Google Scholar] [CrossRef]





| Target Peptide or Protein | Primary Antibody | Secondary Antibody |
|---|---|---|
| Pigment-dispersing factor (PDF) | Rabbit anti-Gryllus PDF antibody for single immunohistochemistry (RRID:AB_2916037, from Dr. Tomioka) 1:1000 | Donkey anti-Rabbit IgG (H + L) Secondary Antibody, TRITC (A-16028, Invitrogen) 1:1000 |
| Mouse anti-Drosophila PDF antibody for double staining with forward-fill (RRID:AB_760350, DSHB PDF C7) 1:100 | Goat anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 (A-21235, Invitrogen) 1:1000 | |
| Embryonic lethal abnormal vision (ELAV) | Rat anti-Drosophila ELAV antibody (RRID:AB_528218, DSHB Rat-ELAV-7E8A10 anti-ELAV) 1:200 | Goat anti-Rat IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 (A-21247, Invitrogen) 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, K.; Shiga, S. Bolwig Organ and Its Role in the Photoperiodic Response of Sarcophaga similis Larvae. Insects 2023, 14, 115. https://doi.org/10.3390/insects14020115
Hirata K, Shiga S. Bolwig Organ and Its Role in the Photoperiodic Response of Sarcophaga similis Larvae. Insects. 2023; 14(2):115. https://doi.org/10.3390/insects14020115
Chicago/Turabian StyleHirata, Kazuné, and Sakiko Shiga. 2023. "Bolwig Organ and Its Role in the Photoperiodic Response of Sarcophaga similis Larvae" Insects 14, no. 2: 115. https://doi.org/10.3390/insects14020115