Effects of Pollen Deprivation in Groups of Tellian (Apis mellifera intermissa) and Saharan (Apis mellifera sahariensis) Honey Bees under Controlled Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
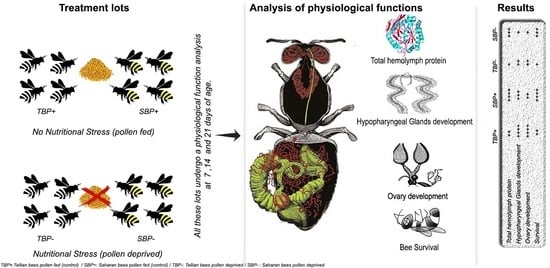
2. Materials and Methods
2.1. Source of the Honeybees
2.2. Average Weight of Emerging Bees
2.3. Experimental Design
2.4. Hemolymph Extraction
2.5. Total Protein
2.6. Extraction and Measurement of Hypopharyngeal Glands (HPG)
2.7. Ovary Assessments
2.8. Statistical Analysis
3. Results
3.1. Average Weight of Emerging Bees
3.2. Food Consumption
3.3. Total Hemolymph Protein
3.4. Hypopharyngeal Glands’ Development
3.5. Ovary Development
3.6. Bee Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, F.; Wallberg, A.; Webster, M.T. From where did the western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Ruttner, F. Biogeography and Taxonomy of Honey Bees; Springer: Heidelberg, Germany, 1988. [Google Scholar]
- Sheppard, W.S.; Meixner, M.D. Apis mellifera pomonella, a new honey bee subspecies from Central Asia. Apidologie 2003, 34, 367–375. [Google Scholar] [CrossRef]
- Haccour, P. Recherches sur l’abeille saharienne au Maroc. Belg. Apic 1961, 25, 13–18. [Google Scholar]
- Mallek, S.; Ladjali, K.; Mohammedi, A. Les Menaces Naturelles et Anthropiques Mettant en Peril l’Existence de l’Abeille Saharienne en Algerie; Bulletin de la Société d‘Histoire Naturelle d‘Afrique du Nord, USTHB: Alger, Algeria, 2018; Volume 74, pp. 165–196. [Google Scholar]
- Le Conte, Y.L.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Rev. Sci. Tech. 2008, 27, 499–510. [Google Scholar]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen protein: Lipid Macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.E.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2015, 47, 15–25. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Corby-Harris, V.; Carroll, M.; Toth, A.L.; Gage, S.; Watkins deJong, E.; Graham, H.; Chambers, M.; Meador, C.; Obernesser, B. The importance of time and place: Nutrient composition and utilization of seasonal pollens by european honey bees (Apis mellifera L.). Insects 2021, 12, 235. [Google Scholar] [CrossRef]
- Pernal, S.F.; Currie, R.W. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 2000, 31, 387–409. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Higo, H.A.; Winston, M.L. Worker honey bee ovary development: Seasonal variation and the influence of larval and adult nutrition. J. Comp. Physiol. B 2006, 176, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Fitz, W.; Copeland, D.C.; Mott, B.M.; Maes, P.; Floyd, A.S.; Dockstader, A.; Anderson, K.E. The impact of pollen consumption on honey bee (Apis mellifera) digestive physiology and carbohydrate metabolism. Arch. Insect Biochem. Physiol. 2017, 96, e21406. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, A.F.; Winston, M.L. The Influence of pollen storage area and Varroa jacobsoni Oudemans parasitism on temporal caste structure in honey bees (Apis mellifera L.). Insectes Soc. 2000, 47, 177–182. [Google Scholar] [CrossRef]
- Kunert, K.; Crailsheim, K. Seasonal changes in carbohydrate, lipid and protein content in emerging worker honeybees and their mortality. J. Apic. Res. 1988, 27, 13–21. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W. Effects of pollen availability and Nosema infection during the spring on division of labor and survival of worker honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2006, 35, 708–717. [Google Scholar] [CrossRef]
- Rueppell, O.; Page, R.E.; Fondrk, M.K. Male behavioural maturation rate responds to selection on pollen hoarding in honeybees. Anim. Behav. 2006, 71, 227–234. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. Costs of environmental fluctuations and benefits of dynamic decentralized foraging decisions in honey bees. Adapt. Behav. 2004, 12, 263–277. [Google Scholar] [CrossRef]
- Crailsheim, K.; Stolberg, E. Influence of diet, age and colony condition upon intestinal proteolytic activity and size of the hypopharyngeal glands in the honeybee (Apis mellifera L.). J. Insect Physiol. 1989, 35, 595–602. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Louveaux, J.; Albisetti, M.; Delangue, M.; Theurkauff, M. Les modalités de l’adaptation des abeilles (Apis mellifica) au milieu naturel. Ann. Abeille 1966, 9, 323–350. [Google Scholar] [CrossRef]
- Shaibi, T.; Moritz, R.F.A. 10,000 Years in isolation? honeybees (Apis mellifera) in saharan oases. Conserv. Genet. 2010, 11, 2085–2089. [Google Scholar] [CrossRef]
- Pain, J. Note technique nouveau modèle de cagtte expérimentales pour le maintien d’abeilles en capativité. Ann. Abeille 1966, 9, 71–76. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker Honey Bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Hess, G. Ueber den Einfluss der Weisellosigkeit und des Fruchtbarkeitsvitamins E auf die Ovarien der Bienenarbeiterin: Ein Beitrag zur Frage der Regulationen im Bienenstaat. Ph.D. Thesis, Eidgenössischen Technischen Hochschule Zürich, Zürich, Switzerland, 1942. [Google Scholar]
- Velthuis, H.H.W. Ovarian development in Apis mellifera worker bees. Entomol. Exp. Appl. 1970, 13, 377–394. [Google Scholar] [CrossRef]
- Zakaria, M.E. Factors affecting on the food metabolism in some honey bee races. J. Appl. Sci. Res. 2007, 3, 311–316. [Google Scholar]
- Poiani, S.B.; da Cruz-Landim, C. Storaged products and presence of acid phosphatase in fat body cells at pre-pupal worker stage of Apis mellifera Linnaeus, 1758 (Hymenoptera, Apidae). Micron 2012, 43, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Cappelari, F.A.; Turcatto, A.P.; Morais, M.M.; De Jong, D. Africanized honey bees more efficiently convert protein diets into hemolymph protein than do Carniolan bees (Apis mellifera carnica). Genet. Mol. Res. 2009, 8, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.W.T.; Howes, C.G.; Foster, L.J. Quantitative comparison of caste differences in honeybee hemolymph. Mol. Cell Proteom. 2006, 5, 2252–2262. [Google Scholar] [CrossRef]
- Vierstraete, E.; Verleyen, P.; Baggerman, G.; D’Hertog, W.; Van den Bergh, G.; Arckens, L.; De Loof, A.; Schoofs, L. A proteomic approach for the analysis of instantly released wound and immune proteins in Drosophila melanogaster hemolymph. Proc. Natl. Acad. Sci. USA 2004, 101, 470–475. [Google Scholar] [CrossRef]
- Free, J.B. Hypopharyngeal gland development and division of labour in hony-bee (Apis mellifera L.) Colonies. Proc. R. Entomol. Soc. Lond. Ser. Gen. Entomol. 1961, 36, 5–8. [Google Scholar] [CrossRef]
- Knecht, D.; Kaatz, H.H. Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie 1990, 21, 457–468. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Boodhoo, C.; Human, H.; Nicolson, S.W. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 2010, 41, 62–72. [Google Scholar] [CrossRef]
- Ruttner, F.; Hesse, B. Rassenspezifische unterschiede in ovarentwicklung und eiablage von weisellosen arbeiterinnen der honibiene Apis mellifera L. Apidologie 1981, 12, 159–183. [Google Scholar] [CrossRef]
- Hepburn, H.R.; Radloff, S.E. Honeybees of Africa; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-642-08389-1. [Google Scholar]
- Gregorc, A.; Sampson, B.; Knight, P.R.; Adamczyk, J. Diet quality affects honey bee (Hymenoptera: Apidae) mortality under laboratory conditions. J. Apic. Res. 2019, 58, 492–493. [Google Scholar] [CrossRef]
- Fluri, P.; Lüscher, M.; Wille, H.; Gerig, L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 1982, 28, 61–68. [Google Scholar] [CrossRef]
- Seehuus, S.-C.; Norberg, K.; Krekling, T.; Fondrk, K.; Amdam, G.V. Immunogold localization of vitellogenin in the ovaries, hypopharyngeal glands and head fat bodies of honeybee workers, Apis mellifera. J. Insect Sci. 2007, 7, 1–14. [Google Scholar] [CrossRef]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey Bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Omholt, S.W.; Kryger, P.; Lourenço, A.P.; Bitondi, M.M.G.; Simões, Z.L.P. Higher vitellogenin concentrations in honey bee workers may be an adaptation to life in temperate climates. Insect. Soc. 2005, 52, 316–319. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.P.; Arne, H.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Koubová, J.; Sábová, M.; Brejcha, M.; Kodrík, D.; Čapková Frydrychová, R. Seasonality in telomerase activity in relation to cell size, DNA replication, and nutrients in the fat body of Apis mellifera. Sci. Rep. 2021, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Stindl, R.; Stindl, W. Vanishing honey bees: Is the dying of adult worker bees a consequence of short telomeres and premature aging? Med. Hypotheses 2010, 75, 387–390. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]




| Mean Survival ± Standard Error | ||
|---|---|---|
| Diet | Apis mellifera sahariensis | Apis mellifera intermissa |
| With pollen | 27.50 ± 0.78 a | 16.76 ± 0.41 c |
| Without pollen | 22.31 ± 0.50 b | 15.33 ± 0.36 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedidji, H.; Abderrahmani, K.; Oulebsir-Mohandkaci, H.; Ladjali-Mohammedi, K.; Mohammedi, A. Effects of Pollen Deprivation in Groups of Tellian (Apis mellifera intermissa) and Saharan (Apis mellifera sahariensis) Honey Bees under Controlled Conditions. Insects 2022, 13, 727. https://doi.org/10.3390/insects13080727
Khedidji H, Abderrahmani K, Oulebsir-Mohandkaci H, Ladjali-Mohammedi K, Mohammedi A. Effects of Pollen Deprivation in Groups of Tellian (Apis mellifera intermissa) and Saharan (Apis mellifera sahariensis) Honey Bees under Controlled Conditions. Insects. 2022; 13(8):727. https://doi.org/10.3390/insects13080727
Chicago/Turabian StyleKhedidji, Hassiba, Khaled Abderrahmani, Hakima Oulebsir-Mohandkaci, Kafia Ladjali-Mohammedi, and Arezki Mohammedi. 2022. "Effects of Pollen Deprivation in Groups of Tellian (Apis mellifera intermissa) and Saharan (Apis mellifera sahariensis) Honey Bees under Controlled Conditions" Insects 13, no. 8: 727. https://doi.org/10.3390/insects13080727