A Binary Mixture of Emamectin Benzoate and Chlorantraniliprole Supplemented with an Adjuvant Effectively Controls Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Insecticides
2.2. Diet-Incorporated Bioassay
2.3. Leaf-Spray Bioassay
2.4. Field Trial
2.5. Statistical Analysis
3. Results
3.1. Synergistic Effect of the EB × CT Mixture
3.2. Synergistic Effect of Adjuvant JIJIAN® on Insecticides and the EB × CT Mixture
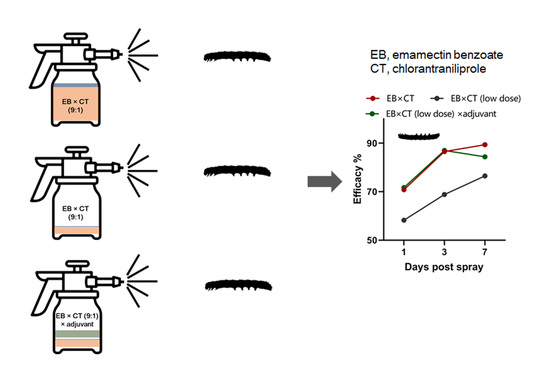
3.3. Promising Efficacy of EB × CT × Jijian® in the Field Trial
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montezano, D.G.; Specht, A.; Sosa-Gomez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Romeis, J. Managing the invasive fall armyworm through biotech crops: A Chinese perspective. Trends Biotechnol. 2021, 39, 105–107. [Google Scholar] [CrossRef]
- Gao, X.W. Current status and development strategy for chemical control in China. Plant Prot. 2010, 36, 19–22. (In Chinese) [Google Scholar] [CrossRef]
- Wu, K.M. Development direction of crop pest control science and technology in China. J. Agric. 2018, 8, 35–38. (In Chinese) [Google Scholar]
- Wu, K.M. Managment strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Paredes-Sanchez, F.A.; Rivera, G.; Bocanegra-Garcia, V.; Martinez-Padron, H.Y.; Berrones-Morales, M.; Nino-Garcia, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A Review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef]
- MARA. MARA on the Issuance of the “2020 National Plan for the Prevention and Control of Fall Armyworm”. Available online: http://www.moa.gov.cn/xw/bmdt/202002/t20200221_6337551.htm (accessed on 30 September 2022).
- Gutierrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Teran-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Huang, J.M.; Ni, H.; Guo, D.; Yang, F.X.; Wang, X.; Wu, S.F.; Gao, C.F. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E. Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef]
- Shi, T.; Tang, P.; Wang, X.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of nicotinic acetylcholine receptor (nAChR) alpha6 subunit confers high levels of resistance to spinosyns in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2022, 187, 105191. [Google Scholar] [CrossRef]
- Gui, F.R.; Lan, T.M.; Zhao, Y.; Guo, W.; Dong, Y.; Fang, D.M.; Liu, H.; Li, H.M.; Wang, H.L.; Hao, R.S.; et al. Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 2022, 13, 513–531. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhao, R.; Gao, J.; Zhang, L.; Zhang, S.; Liang, P.; Gao, X.W.; Gu, S.H. Genetic architecture and insecticide resistance in Chinese populations of Spodoptera frugiperda. J. Pest. Sci. 2022. [Google Scholar] [CrossRef]
- Lv, S.L.; Shi, Y.; Zhang, J.C.; Liang, P.; Zhang, L.; Gao, X.W. Detection of ryanodine receptor target-site mutations in diamide insecticide-resistant Spodoptera frugiperda in China. Insect Sci 2021, 28, 639–648. [Google Scholar] [CrossRef]
- Li, Q.; Jin, M.H.; Yu, S.M.; Cheng, Y.; Shan, Y.X.; Wang, P.; Yuan, H.B.; Xiao, Y.T. Knockout of the ABCB1 gene increases susceptibility to emamectin benzoate, beta-cypermethrin and chlorantraniliprole in Spodoptera frugiperda. Insects 2022, 13, 137. [Google Scholar] [CrossRef]
- Liu, J.B.; Hao, Z.; Yang, S.Y.; Lin, Y.Y.; Zhong, H.; Jin, T. Insecticide resistance and its underlying synergism in field populations of Spodoptera frugiperda (J.E. Smith) from Hainan Island, China. Phytoparasitica 2022, 50, 933–945. [Google Scholar] [CrossRef]
- Muraro, D.S.; Neto, D.D.A.; Kanno, R.H.; Kaiser, I.S.; Bernardi, O.; Omoto, C. Inheritance patterns, cross-resistance and synergism in Spodoptera frugiperda (Lepidoptera: Noctuidae) resistant to emamectin benzoate. Pest. Manag. Sci. 2021, 77, 5049–5057. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gao, Q.; Cao, H. Synergistic effect of spinosad and indoxacarb mixture on Spodoptera frugiperda (J.E. Smith). Plant Prot. 2020, 46, 276–278. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Q.; Yang, S.; Zhang, B. Toxicities and field control efficacy of the mixtures of emamectin benzoate and tetrachlorantraniliprole of Spodoptera frugiperda. Agrochemicals 2021, 60, 306–309. (In Chinese) [Google Scholar]
- Zhao, S.Y.; Sun, X.X.; Zhang, H.W.; Yang, X.M.; Wu, K.M. Laboratory test on the control efficacy of common chemical insecticides against Spodoptera frugiperda. Plant Prot. 2019, 45, 10–14. (In Chinese) [Google Scholar] [CrossRef]
- Hardke, J.T.; Temple, J.H.; Leonard, B.R.; Jackson, R.E. Laboratory toxicity and field efficacy of selected insecticides against fall armyworm (Lepidoptera: Noctuidae). Fla. Entomol. Soc. 2011, 94, 272–278. [Google Scholar] [CrossRef]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- LeOra Software. POLO-Plus 1.0 Probit and Logit Analysis; LeOra Software: Petaluma, CA, USA, 2006. [Google Scholar]
- Sun, Y.P.; Johnson, E.R. Analysis of joint action of insecticides against house flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Zhang, D.D.; Xiao, Y.T.; Xu, P.J.; Yang, X.M.; Wu, Q.L.; Wu, K.M. Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J. Integr. Agric. 2021, 20, 783–791. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Hu, F.; Su, X.; Hu, B.; Zhou, Z.; Xu, L.; Qiu, K.; Zheng, Z.; Zhang, Q.; Liao, C.; Wang, Z. Toxicities and field control efficacy of the mixtures of emamectin benzoate and chlorantraniliprole to Spodoptera frugiperda. Plant Prot. 2020, 46, 303–307. (In Chinese) [Google Scholar] [CrossRef]
- Feng, C.; Yin, H.; Yang, R.; Xiong, J.; Tang, B.; Zhang, J.; Yu, Y. Effect of Jijian in pesticide reduction and pest controlling in rice field. China Plant Prot. 2016, 36, 61–64. (In Chinese) [Google Scholar]
- Fang, L.; Luo, W.; Liao, H.; Xu, X.; Wang, Z. The application of Jijian in controlling pests. Sichuan Agric. Technol. 2022, 9, 47–49. (In Chinese) [Google Scholar]
- Bai, X.; Wang, F.; Fu, Z. Study on the pesticide reduction effect of Jijian in controlling Ostrinia furnacalis. Mod. Agric. Technol. 2019, 13, 98. (In Chinese) [Google Scholar]

| Pesticides a | Mass Ratio (EB:CT) | LC50 (mg/L) | 95% Confidential Limit | Slope ± SE | Chi-Square | ATIM b | TTIM b | CTC b |
|---|---|---|---|---|---|---|---|---|
| EB | / | 0.383 | 0.280~0.500 | 1.853 ± 0.273 | 1.950 | / | / | / |
| CT | / | 9.703 | 5.906~15.012 | 1.169 ± 0.243 | 1.118 | / | / | / |
| EB × CT | 1:9 | 5.390 | 3.305~10.114 | 1.259 ± 0.296 | 0.341 | 7.11 | 13.55 | 52.43 |
| 3:7 | 0.908 | 0.631~1.348 | 1.662 ± 0.314 | 2.659 | 42.18 | 32.76 | 128.74 | |
| 5:5 | 0.620 | 0.497~0.773 | 2.381 ± 0.305 | 2.603 | 61.77 | 51.97 | 118.86 | |
| 7:3 | 0.659 | 0.498~0.857 | 2.146 ± 0.322 | 0.587 | 58.12 | 70.18 | 81.65 | |
| 9:1 | 0.177 | 0.129~0.231 | 2.354 ± 0.410 | 0.191 | 216.38 | 90.39 | 239.38 |
| Insecticides or Mixture a | LC50 (mg/L) | 95% Confidential Limit | Slope ± SE | Chi-Square | ATIM b | TTIM b | CTC b |
|---|---|---|---|---|---|---|---|
| EB | 0.842 | 0.678~1.080 | 2.259 ± 0.303 | 2.213 | / | / | / |
| EB × adjuvant | 0.310 | 0.195~0.544 | 1.832 ± 0.258 | 3.569 | / | / | / |
| CT | 35.173 | 26.580~45.94 | 1.711 ± 0.250 | 0.754 | / | / | / |
| CT × adjuvant | 14.200 | 11.364~17.765 | 2.413 ± 0.368 | 1.193 | / | / | / |
| EB × CT (9:1) | 0.482 | 0.380~0.609 | 2.039 ± 0.269 | 2.213 | 174.69 | 90.24 | 193.58 |
| EB × CT (9:1) × adjuvant | 0.197 | 0.151~0.252 | 1.903 ± 0.262 | 3.569 | 157.36 | 90.22 | 174.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, J.; Wang, K.; Zhang, Y.; Liu, Z.; Yu, N. A Binary Mixture of Emamectin Benzoate and Chlorantraniliprole Supplemented with an Adjuvant Effectively Controls Spodoptera frugiperda. Insects 2022, 13, 1157. https://doi.org/10.3390/insects13121157
Zhang J, Jiang J, Wang K, Zhang Y, Liu Z, Yu N. A Binary Mixture of Emamectin Benzoate and Chlorantraniliprole Supplemented with an Adjuvant Effectively Controls Spodoptera frugiperda. Insects. 2022; 13(12):1157. https://doi.org/10.3390/insects13121157
Chicago/Turabian StyleZhang, Junteng, Jianjun Jiang, Kan Wang, Yixi Zhang, Zewen Liu, and Na Yu. 2022. "A Binary Mixture of Emamectin Benzoate and Chlorantraniliprole Supplemented with an Adjuvant Effectively Controls Spodoptera frugiperda" Insects 13, no. 12: 1157. https://doi.org/10.3390/insects13121157