Ecological Aspects of the Phlebotominae Fauna (Diptera: Psychodidae) among Forest Fragments and Built Areas in an Endemic Area of American Visceral Leishmaniasis in João Pessoa, Paraíba, Brazil
Abstract
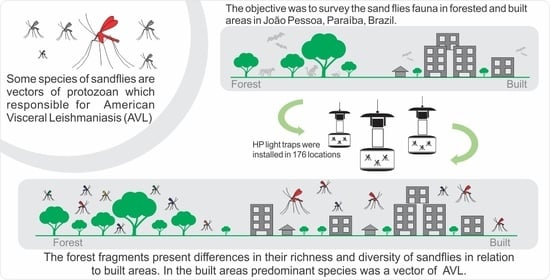
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Entomological Captures and Identification
2.3. Survey of Environmental Conditions
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimabukuro, P.H.F.; de Andrade, A.J.; Galati, E.A.B. Checklist of American sand flies (Diptera, Psychodidae, Phlebotominae): Genera, species, and their distribution. Zookeys 2017, 660, 67–106. [Google Scholar] [CrossRef] [Green Version]
- Brazil, R.P.; Brazil, B.G. Bionomy: Biology of Neotropical Phlebotomine Sand Flies. In Brazilian Sand Flies; Rangel, E.F., Lainson, R., Eds.; Springer International Publishing AG, part of Springer Nature: Cham, Switzerland, 2018; pp. 299–318. [Google Scholar]
- Shaw, J.; Rosa, A.; Ribeiro Cruz, A.C.; Vasconcelos, P. Brazilian Phlebotomines as Hosts and Vectors of Viruses, Bacteria, Fungi, Protozoa (Excluding Those Belonging to the Genus Leishmania) and Nematodes. In Brazilian Sand Flies; Rangel, E.F., Lainson, R., Eds.; Springer International Publishing AG, part of Springer Nature: Cham, Switzerland, 2018; pp. 417–441. [Google Scholar]
- Ovallos, F.; Silva, R.; Gusmon, V.; Sabio, P.; Galati, E. Leishmaniose no Brasil: Aspectos epidemiológicos, desafios e perspectivas In Atualidades em Medicina Tropical No Brasil: Protozoários; Stricto Sensu: Rio Branco, Brazil, 2020; pp. 227–255. [Google Scholar]
- Galati, E. Morfologia e terminologia de Phlebotominae (Diptera: Psychodidae). Classificação e identificação de táxons das Américas [Internet]. Volume I. Faculdade de Saúde Pública da Universidade de São Paulo, São Paulo, 2021. Available online: https://www.fsp.usp.br/egalati/wp-content/uploads/2021/10/Nova_Apostila_Vol-I_2021.pdf (accessed on 25 July 2022).
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 12 July 2022).
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization/World Health Organization (PAHO/WHO). Manual de Procedimientos para Vigilancia y Control de las Leishmaniasis en las Américas; OPAS: Washington, DC, USA, 2019; pp. 12–26. [Google Scholar]
- Lima-Junior, M.; de Almeida, P.S.; da Silva, J.O.; da Silva, R.A.; Faccenda, O.; de Aquino Coelho, D.; Costa, G.B.; de Souza, A.R.; Fernandes, M.G.; Neitzke-Abreu, H.C. Sand Fly Fauna, Spatial Distribution of Lutzomyia longipalpis (Diptera: Psychodidae), and Climate Factors in Dourados, Brazil. J. Med. Entomol. 2021, 58, 1952–1957. [Google Scholar] [CrossRef]
- Ministério da Saúde. Secretaria de Vigilância em Saúde. In Manual de Vigilância e Controle da Leishmaniose Visceral, 1st ed.; Ministério da Saúde: Brasília, Brazil, 2014; p. 120. [Google Scholar]
- Gontijo, C.M.F.; Melo, M.N. Leishmaniose visceral no Brasil: Quadro atual, desafios e perspectivas. Rev. Bras. Epidemiol. 2004, 7, 338–349. [Google Scholar] [CrossRef]
- Lima, R.G.d.; Mendonça, T.M.; Mendes, T.d.S.; Menezes, M.V.C. Perfil epidemiológico da leishmaniose visceral no Brasil, no período de 2010 a 2019. REAS 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Salomon, O.D.; Feliciangeli, M.D.; Quintana, M.G.; Afonso, M.M.; Rangel, E.F. Lutzomyia longipalpis urbanisation and control. Mem. Inst. Oswaldo Cruz 2015, 110, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.d.; Perez, L.P.; Oliveira, B.F.A.d.; Jacobson, L.d.S.V.; Horta, M.A.; Sobral, A.; Hacon, S.d.S. Vector-borne diseases in Brazil: Climate change and future warming scenarios. Sustain. Debate 2020, 11, 361–404. [Google Scholar] [CrossRef]
- Rangel, E.F.; Lainson, R.; Carvalho, B.M.; Costa, S.M.; Shaw, J.J. Sand Fly Vectors of American Cutaneous Leishmaniasis in Brazil. In Brazilian Sand Flies; Rangel, E.F., Lainson, R., Eds.; Springer International Publishing AG, part of Springer Nature: Cham, Switzerland, 2018; pp. 341–380. [Google Scholar]
- Afonso, M.M.d.S.; Chaves, S.A.d.M.; Magalhães, M.d.A.F.M.; Gracie, R.; Azevedo, C.; Carvalho, B.M.; Rangel, E.F. Ecoepidemiology of American Visceral Leishmaniasis in Tocantins State, Brazil: Factors Associated with the Occurrence and Spreading of the Vector Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae). In The Epidemiology and Ecology of Leishmaniasis; Claborn, D., Ed.; IntechOpen: London, UK, 2017; pp. 91–115. [Google Scholar]
- Alencar, J.; Mello, C.F.; Serra-Freire, N.M.; Guimaraes, A.E.; Gil-Santana, H.R.; Gleiser, R.M. Biodiversity and Temporal Distribution of Immature Culicidae in the Atlantic Forest, Rio de Janeiro State, Brazil. PLoS ONE 2016, 11, e0159240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Cornuault, J.; Gantier, J.C.; Manzi, S.; Chavy, A.; Girod, R.; Dusfour, I.; Forget, P.M.; Ginouves, M.; Prevot, G.; et al. Biodiversity and vector-borne diseases: Host dilution and vector amplification occur simultaneously for Amazonian leishmaniases. Mol. Ecol. 2022. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap—Surveillance mondiale de la leishmaniose: 2019–2020, une période de référence pour la feuille de route à l’horizon 2030. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire. Wkly. Epidemiol. Rec. 2021, 96, 401–419. [Google Scholar]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar] [PubMed]
- Koch, L.K.; Kochmann, J.; Klimpel, S.; Cunze, S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci. Rep. 2017, 7, 13325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva Neto, A.B.D.; Oliveira, E.F.; Encina, C.C.C.; Figueiredo, H.R.; Paranhos Filho, A.C.; Oliveira, A.G. Effects of El Nino-Southern Oscillation on human visceral leishmaniasis in the Brazilian State of Mato Grosso do Sul. Mem. Inst. Oswaldo Cruz 2020, 115, e190298. [Google Scholar] [CrossRef]
- Santos, C.; Seva, A.D.P.; Werneck, G.L. Does deforestation drive visceral leishmaniasis transmission? A causal analysis. Proc. Biol. Sci. 2021, 288, 20211537. [Google Scholar] [CrossRef] [PubMed]
- Arzamani, K.; Vatandoost, H.; Rassi, Y.; Akhavan, A.A.; Abai, M.R.; Alavinia, M.; Akbarzadeh, K.; Mohebali, M.; Rafizadeh, S. Richness and Diversity of Phlebotomine Sand Flies (Diptera: Psychodidae) in North Khorasan Province, Northeast of Iran. J. Arthropod-Borne Dis. 2018, 12, 232–239. [Google Scholar] [CrossRef]
- Rangel, E.F.; Lainson, R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: Aspects of their biology and vectorial competence. Mem. Inst. Oswaldo Cruz 2009, 104, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Manual de Vigilância e Controle da Leishmaniose Viscera, 1st ed.; Ministério da Saúde: Brasília, Brazil, 2014.
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: A review. Mem. Inst. Oswaldo Cruz 2005, 100, 811–827. [Google Scholar] [CrossRef]
- Carvalho, M.R.d.; Valenca, H.F.; da Silva, F.J.; de Pita-Pereira, D.; de Araujo Pereira, T.; Britto, C.; Brazil, R.P.; Brandao Filho, S.P. Natural Leishmania infantum infection in Migonemyia migonei (Franca, 1920) (Diptera:Psychodidae:Phlebotominae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Acta Trop. 2010, 116, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.O.d.; Arias, J.; Ribeiro, A.A.; Paiva, M.H.; Freitas, R.A.; Malacco, M.A. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis. Med. Vet. Entomol. 1998, 12, 315–317. [Google Scholar] [CrossRef]
- Deane, M.P.; Deane, L.M. Natural infection of Phlebotomus longipalpis by Leptomonas, probably Leishmania donovani, in a focus of kala-azar in Ceará. Hospital 1954, 45, 697–702s. [Google Scholar]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.A.; Nunes, V.L.; Rego Junior Fde, A.; Oshiro, E.T.; Chang, M.R. Phlebotomines (Diptera: Psychodidae) focusing visceral leishmaniasis in the State of Mato Grosso do Sul, Brazil. Rev. Saude Publica 1997, 31, 378–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcao, E.O.; Oshiro, E.T.; Fernandes, W.S.; Murat, P.G.; Medeiros, M.J.; Souza, A.I.; Oliveira, A.G.; Galati, E.A. Experimental infection and transmission of Leishmania by Lutzomyia cruzi (Diptera: Psychodidae): Aspects of the ecology of parasite-vector interactions. PLoS Negl. Trop. Dis. 2017, 11, e0005401. [Google Scholar]
- Guimaraes, V.C.; Pruzinova, K.; Sadlova, J.; Volfova, V.; Myskova, J.; Filho, S.P.; Volf, P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit. Vectors 2016, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Organização Pan-Americada da Saúde. Leishmanioses: Informe Epidemiológico nas Américas; OPAS: Washington, DC, USA, 2021. [Google Scholar]
- Governo da Paraíba. Secretaria de Estado da Saúde. Plano Estadual de Ação para Intensificação da Vigilância e Controle de Leishmaniose Visceral; Governo da Paraíba: Paraíba, Brazil, 2019; p. 16.
- Ministério da Saúde. Secretaria de Vigilância em Saúde. In Guia de Vigilância em Saúde [Recurso Eletrônico], 5th ed.; Ministério da Saúde: Brasília, Brazil, 2022. [Google Scholar]
- Miranda, D.E.; Sales, K.G.; Faustino, M.A.; Alves, L.C.; Brandao-Filho, S.P.; Dantas-Torres, F.; de Carvalho, G.A. Ecology of sand flies in a low-density residential rural area, with mixed forest/agricultural exploitation, in north-eastern Brazil. Acta Trop. 2015, 146, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.P.; Silva, M.M.; Silva Junior, J.B.; da Silva, J.H.; Alves Mde, L.; Ximenes Mde, F. Sand flies (Diptera, Psychodidae, Phlebotominae), vectors of Leishmania protozoa, at an Atlantic Forest Conservation Unit in the municipality of Nisia Floresta, Rio Grande do Norte state, Brazil. Parasit. Vectors 2016, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.L.M.; Marinho, H.T.S.; Conceicao, M.A.B.; Macario, J.M.R. Effects of forest degradation on the sand fly communities of northeast Brazil. J. Vector Ecol. 2020, 45, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Guedes, G.E.; Maroja, A.; Chaves, E.; Estélio, J.; Cunha, M.J.; Arcoverde, S. Calazar no estado da Paraíba, Brasil, encontro de 70 casos humanos e 16 caninos. Rev. Inst. Med. Trop. 1974, 16, 265–269. [Google Scholar]
- Silva, F.A. Avaliação da Dinâmica dos Vetores de Leishmania spp. em Fragmentos de Mata Atlântica e em Ambiente Urbano: Influência dos Fatores Climáticos e Ambientais e Suas Interferências na Saúde Pública. Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil, 2018. [Google Scholar]
- BGE—Instituto Brasileiro de Geografia Estatística. 2022. Available online: https://cidades.ibge.gov.br/brasil/pb/joao-pessoa/panorama (accessed on 17 February 2022).
- Koeppen, W. Das Geographische System der Klimate, Handbuch der Klimatologie [The Geographical System of the Climate, Handbook of Climatology]; Teil. C.: Berlin, Germany, 1936; Volume 1. [Google Scholar]
- Souza, J.F.d.; Silva, R.M.; Silva, A.M. Influência do uso e ocupação do solo na temperatura da superfície: O estudo de caso de João Pessoa—PB. Ambient. Construído 2016, 16, 21–37. [Google Scholar] [CrossRef] [Green Version]
- SEMAM—Secretaria Municipal de Meio Ambiente. Plano Municipal de Conservação e Recuperação da Mata Atlântica. Prefeitura Muncipal de João Pessoa. 2010. Available online: https://docplayer.com.br/16714724-Plano-municipal-de-conservacao-e-recuperacao-da-mata-atlantica.html (accessed on 3 August 2022).
- Empresa Paraibana de Turismo. Indicadores de Turismo. Fluxo Global Estimado de Turismo 2009–2022. 2022. Available online: https://www.pbtur.pb.gov.br/wp-content/uploads/2022/03/FluxoGlobalPBJaneiro2022.pdf (accessed on 10 August 2022).
- SUDEMA—Superintendência de Administração do Meio Ambiente. Estudo para Subsidiar a Criação de Unidade de Conservação de Proteção Integral da Mata do Buraquinho, Paraíba; SUDEMA: Paraíba, Brazil, 2014; p. 134. [Google Scholar]
- Brito, E.F.A.; Vanzella, E. Jardim Botânico Benjamim Maranhão: Contribuições para a Cidade de João Pessoa. Rev. Mangaio Acad. 2018, 2, 7–14. [Google Scholar]
- Bizerra, D.d.S. Dinâmica Físico-Ambiental no Parque Estadual Mata de Jacarapé. Bachelor’s Thesis, University of Paraíba, João Pessoa, Paraíba, Brazil, 2013. [Google Scholar]
- Pugedo, H.; Barata, R.A.; Franca-Silva, J.C.; Silva, J.C.; Dias, E.S. HP: An improved model of suction light trap for the capture of small insects. Rev. Soc. Bras. Med. Trop. 2005, 38, 70–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, M.L.; Zwetsch, A.; Silva, J.d.S. Methods for Capturing, Processing and Preserving Phlebotominae. In Brazilian Sand Flies; Rangel, E.F., Lainson, R., Eds.; Springer International Publishing AG, part of Springer Nature: Cham, Switzerland, 2018; pp. 443–466. [Google Scholar]
- Marcondes, C.B. A Proposal of Generic and Subgeneric Abbreviations for Phlebotomine Sandflies (Diptera: Psychodidae: Phlebotominae) of the World. Entomol. News 2007, 118, 351–356. [Google Scholar] [CrossRef]
- INMET—Instituto Nacional de Metereologia. 2022. Available online: https://tempo.inmet.gov.br/TabelaEstacoes/A001 (accessed on 10 January 2022).
- Rényi, A. On Measures of Entropy and Information. In Proceedings of the 4th Berkeley Symposium on Mathematics, Statistics and Probability, Berkeley, CA, USA, 20 June–30 July 1960; University of California Press: Californi, CA, USA, 1961; Volume 1, pp. 547–561. [Google Scholar]
- Belen, A.; Alten, B. Seasonal dynamics and altitudinal distributions of sand fly (Diptera: Psychodidae) populations in a cutaneous leishmaniasis endemic area of the Cukurova region of Turkey. J. Vector Ecol. 2011, 36 (Suppl. S1), S87–S94. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [PubMed] [Green Version]
- Dancey, C.P.; Reidy, J. Statistics without Maths for Psychology, 5th ed.; Pearson Education: Harlow, UK, 2011; p. 560. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species:The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical: Vienna, Austria, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, P.L.R.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.5-7; R Foundation for Statistical: Vienna, Austria, 2020. [Google Scholar]
- Oliveira, D.M.; Saraiva, E.M.; Ishikawa, E.A.; Sousa, A.A.; Silva, E.O.; Silva, I.M. Distribution of phlebotomine fauna (Diptera: Psychodidae) across an urban-rural gradient in an area of endemic visceral leishmaniasis in northern Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 1039–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahouiti, K.; Lalami, A.E.O.; Maniar, S.; Bekhti, K. African Journal of Environmental Science and Technology Seasonal fluctuations of phlebotomines sand fly populations (Diptera: Psychodidae) in the Moulay Yacoub province, centre Morocco: Effect of ecological factors. Afr. J. Environ. Sci. Technol. 2013, 7, 1028–1036. [Google Scholar]
- Sanguinette Cde, C.; da Silva, D.F.; Stumpp, R.G.; Rego, F.D.; Tonelli, G.B.; Tanure, A.; Gontijo, C.M.; Andrade Filho, J.D. Comparison of the phlebotomine (Diptera: Psychodidae) fauna of urban, transitional, and wild areas in northern Minas Gerais, Brazil. Parasit. Vectors 2015, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Soares, D.C.; Guimarães, R.J.d.P.S.e.; Santos, W.S.; Sousa, G.C.R.d.; Chagas, A.P.; Garcez, L.M. Diversity and ecology of sand flies (Psychodidae: Phlebotominae): Foci of cutaneous leishmaniasis in Amazon Region, Brazil. Rev. Pan. Amaz. Saude 2016, 7, 133–142. [Google Scholar] [CrossRef]
- Martinez, M.F.; Santini, M.S.; Kowalewski, M.M.; Salomon, O.D. Phlebotominae in peri-domestic and forest environments inhabited by Alouatta caraya in northeastern Argentina. Med. Vet. Entomol. 2019, 33, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.W.; Saraiva, L.; Neto, R.G.; Meira, P.C.; Sanguinette Cde, C.; Tonelli, G.B.; Botelho, H.A.; Belo, V.S.; Silva, E.S.; Gontijo, C.M.; et al. Study of sand flies (Diptera: Psychodidae) in visceral and cutaneous leishmaniasis areas in the central-western state of Minas Gerais, Brazil. Acta Trop. 2013, 125, 262–268. [Google Scholar] [CrossRef]
- Pereira, N.C.L.; Michalsky, E.M.; Silva, F.O.L.; Lana, R.S.; Paula, A.J.V.; Pereira, D.M.; Lopes, J.V.; Fortes-Dias, C.L.; Dias, E.S. Ecology of phlebotomine sand flies in a Brazilian area with recent leishmaniasis transmission (Itauna, in Minas Gerais state). Rev. Soc. Bras. Med. Trop. 2020, 53, e20190538. [Google Scholar] [CrossRef]
- Ramos, W.R.; Medeiros, J.F.; Juliao, G.R.; Rios-Velasquez, C.M.; Marialva, E.F.; Desmouliere, S.J.; Luz, S.L.; Pessoa, F.A. Anthropic effects on sand fly (Diptera: Psychodidae) abundance and diversity in an Amazonian rural settlement, Brazil. Acta Trop. 2014, 139, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.F.; Galati, E.A.; Cruz, C.F. Ecological aspects of the sandfly fauna (Diptera, Psychodidae) in an American cutaneous leishmaniasis endemic area under the influence of hydroelectric plants in Paranapanema river, State of Parana, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.C.S.; Marialva, E.F.; Feijo, J.A.; Pereira-Silva, J.W.; Martins-Campos, K.M.; Gontijo, C.M.F.; Pereira, A.A.S.; Rios-Velasquez, C.M.; Pessoa, F.A.C. Trypanosomatids in Phlebotomine Sand Flies (Diptera: Phlebotominae) From Anthropic and Sinantropic Landscapes in a Rural Settlement in the Brazilian Amazon. J. Med. Entomol. 2022, 59, 681–692. [Google Scholar] [CrossRef]
- Souza, C.M.; Pessanha, J.E.; Barata, R.A.; Monteiro, E.M.; Costa, D.C.; Dias, E.S. Study on phlebotomine sand fly (Diptera: Psychodidae) fauna in Belo Horizonte, state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2004, 99, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.F.; Casaril, A.E.; Fernandes, W.S.; Ravanelli, M.S.; Medeiros, M.J.; Gamarra, R.M.; Paranhos Filho, A.C.; Oshiro, E.T.; Oliveira, A.G.; Galati, E.A. Monthly Distribution of Phlebotomine Sand Flies, and Biotic and Abiotic Factors Related to Their Abundance, in an Urban Area to Which Visceral Leishmaniasis Is Endemic in Corumba, Brazil. PLoS ONE 2016, 11, e0165155. [Google Scholar]
- Silva, J.S.; Caranha, L.; Santos, F.K.M.; Santos, A.P.; Silva, L.O.R.; Rangel, E.F. Sand fly (Diptera, Psychodidae, Phlebotominae) abundance and diversity in areas affected by the Sao Francisco River transposition project in Ceara State, Brazil. Parasit. Vectors 2017, 10, 403. [Google Scholar] [CrossRef]
- Oliveira, B.F.G.; de Fatima Domingos, M.; Ovallos, F.G.; de Camargo-Neves, V.L.F. Updating Ecological and Behavioral Aspects of the Sandfly Fauna in the Vale do Ribeira Region, Sao Paulo State, Brazil. Insects 2021, 12, 988. [Google Scholar] [CrossRef]
- Salomon, O.D. Lutzomyia longipalpis, Gone with the Wind and Other Variables. Neotrop. Entomol. 2021, 50, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.C.; Camargo, M.C.; Vieira, J.R.; Nobi, R.C.; Porto, M.N.; Oliveira, C.D.; Pessanha, J.E.; Cunha Mda, C.; Brandao, S.T. Seasonal variation of Lutzomyia longipalpis in Belo Horizonte, State of Minas Gerais. Rev. Soc. Bras. Med. Trop. 2006, 39, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ximenes, M.d.F.F.d.M.; Castellón, E.G.; de Souza, M.d.F.; Menezes, A.A.L.; Queiroz, J.W.; Silva, V.P.M.E.; Jerônimo, S.M.B. Effect of Abiotic Factors on Seasonal Population Dynamics of Lutzomyia longipalpis (Diptera: Psychodidae) in Northeastern Brazil. J. Med. Entomol. 2006, 43, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, M.S.M.; Bavia, M.E.; Fonseca, E.O.L.; Cova, B.O.; Silva, M.M.N.; Carneiro, D.; Cardim, L.L.; Malone, J.B. Ecological niche models for sand fly species and predicted distribution of Lutzomyia longipalpis (Diptera: Psychodidae) and visceral leishmaniasis in Bahia state, Brazil. Environ. Monit. Assess. 2019, 191, 331. [Google Scholar] [CrossRef] [PubMed]
- Cheghabaleki, Z.Z.; Yarahmadi, D.; Karampour, M.; Shamsipour, A. Spatial Dynamics of a Phlebotomine Sand Flies Population in Response to Climatic Conditions in Bushehr Province of Iran. Ann. Glob. Health 2019, 85, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, C.; Andrighetti, M.T.; Sampaio, S.M.; Marcoris, M.L.; Colla-Jacques, F.E.; Prado, A.P. Larval breeding sites of Lutzomyia longipalpis (Diptera: Psychodidae) in visceral leishmaniasis endemic urban areas in Southeastern Brazil. PLoS Negl. Trop. Dis. 2013, 7, e2443. [Google Scholar] [CrossRef]
- Costa, P.L.; Dantas-Torres, F.; Silva, F.J.; Guimaraes, V.C.; Gaudencio, K.; Brandao-Filho, S.P. Ecology of Lutzomyia longipalpis in an area of visceral leishmaniasis transmission in north-eastern Brazil. Acta Trop. 2013, 126, 99–102. [Google Scholar] [CrossRef]
- Pita-Pereira, D.; Alves, C.R.; Souza, M.B.; Brazil, R.P.; Bertho, A.L.; de Figueiredo Barbosa, A.; Britto, C.C. Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridisation assay. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 905–913. [Google Scholar] [CrossRef]
- Ubirajara Filho, C.R.C.; da Silva Sales, K.G.; Lima, T.; Dantas-Torres, F.; Alves, L.C.; de Carvalho, G.A.; Ramos, R.A.N. Lutzomyia evandroi in a New Area of Occurrence of Leishmaniasis. Acta Parasitol. 2020, 65, 716–722. [Google Scholar] [CrossRef]
- Governo do Estado da Paraíba. Secretaria de Estado de Saúde. Casos de Leishmaniose Visceral Americana No Município de João Pessoa de 2010 a 2021; Governo do Estado da Paraíba: João Pessoa, Paraíba, Brazil, 2021.
- Ministério da Saúde; Banco de Dados do Sistema Único de Saúde-DATASUS; Departamento de Informática do SUS. Leishmaniose Tegumentar Americana—Casos Confirmados Notificados no Sistema de Informação de Agravos e Notificação—Paraíba; Ministério da Saúde: Brasilia, Brazil, 2022.
- Oliveira, A.G.; Andrade Filho, J.D.; Falcao, A.L.; Brazil, R.P. Study of sand flies (Diptera, Psychodidae, Phlebotominae) in the urban area of Campo Grande, Mato Grosso do Sul State, Brazil, from 1999 to 2000. Cad. Saude Publica 2003, 19, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcondes, C.B.; Nascimento, J.A. Evaluation of the effectiveness of deltamethrin (K-othrine CE) in the control of Lutzomyia longipalpis (Diptera: Psychodidae), in the municipality of Santa Rita, Paraiba, Brazil. Rev. Soc. Bras. Med. Trop. 1993, 26, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Rangel, E.F.; Vilela, M.L. Lutzomyia longipalpis (Diptera, Psychodidae, Phlebotominae) and urbanization of visceral leishmaniasis in Brazil. Cad. Saude Publica 2008, 24, 2948–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazil, R.P. The dispersion of Lutzomyia longipalpis in urban areas. Rev. Soc. Bras. Med. Trop. 2013, 46, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.; Rossi, C.N. Leishmaniose visceral no Brasil. Braz. J. Vet. Res. Anim. Sci. 2013, 50, 341. [Google Scholar] [CrossRef]




| Species | Females | Males | Total |
|---|---|---|---|
| Lutzomyia longipalpis | 128 | 884 | 1012 |
| Brumptomyia brumpti | 287 | 289 | 576 |
| Evandromyia evandroi | 72 | 113 | 187 |
| Sciopemyia sordellii | 125 | 47 | 172 |
| Psathyromyia brasiliensis | 37 | 68 | 105 |
| Viannamyia furcata | 57 | 6 | 63 |
| Psychodopygus claustrei | 0 | 9 | 9 |
| Migonemyia migonei | 2 | 2 | 4 |
| Brumptomyia sp. | 4 | 0 | 4 |
| Micropygomyia quinquefer | 2 | 0 | 2 |
| Total | 714 | 1424 | 2134 |
| Species | Forest Areas | % Forest | Built Areas | % Built Areas | Total |
|---|---|---|---|---|---|
| Lutzomyia longipalpis | 34 | 3.4% | 978 | 86.1% | 1012 |
| Brumptomyia brumpti | 574 | 57.5% | 2 | 0.2% | 576 |
| Evandromyia evandroi | 38 | 3.8% | 149 | 13.1% | 187 |
| Sciopemyia sordellii | 171 | 17.2% | 1 | 0.1% | 172 |
| Psathyromyia brasiliensis | 100 | 10.0% | 5 | 0.4% | 105 |
| Viannamyia furcata | 63 | 6.3% | 0 | 0 | 63 |
| Psychodopygus claustrei | 9 | 0.9% | 0 | 0 | 9 |
| Migonemyia migonei | 4 | 0.4% | 0 | 0 | 4 |
| Micropygomyia quinquefer | 2 | 0.2% | 0 | 0 | 2 |
| Brumptomyia sp. | 3 | 0.3% | 1 | 0.1% | 4 |
| Total | 998 | 100% | 1136 | 100% | 2134 |
| Species | Area | % | p Value |
|---|---|---|---|
| Lutzomyia longipalpis | Built | 97% | 0.005 |
| Sciopemyia sordellii | Forest | 77% | 0.005 |
| Brumptomyia brumpti | Forest | 74% | 0.005 |
| Viannamyia furcata | Forest | 62% | 0.005 |
| Psathyromyia brasiliensis | Forest | 56% | 0.010 |
| Species | Temperature | Humidity | Precipitation | Wind Speed | ||||
|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | |
| Lutzomyia longipalpis (built) | −0.41 | 0.000 * | 0.80 | 0.000 * | 0.80 | 0.000 * | 0.77 | 0.000 * |
| Evandromyia evandroi (built) | 0.08 | 0.729 | 0.18 | 0.430 | 0.18 | 0.000 * | 0.10 | 0.649 |
| Brumptomyia brumpti (forest) | 0.04 | 0.838 | 0.09 | 0.704 | 0.09 | 0.704 | 0.23 | 0.312 |
| Sciopemyia sordellii (forest) | 0.26 | 0.249 | 0.08 | 0.704 | −0.18 | 0.416 | −0.20 | 0.372 |
| Psathyromyia brasiliensis (forest) | −0.58 | 0.005 * | 0.71 | 0.000 * | 0.71 | 0.000 * | 0.68 | 0.000 * |
| Viannamyia furcata (forest) | −0.47 | 0.031 * | 0.10 | 0.644 | 0.10 | 0.644 | 0.12 | 0.584 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.Q.d.; Afonso, M.M.d.S.; Freire, L.J.M.; Santana, A.L.F.d.; Pereira-Colavite, A.; Rangel, E.F. Ecological Aspects of the Phlebotominae Fauna (Diptera: Psychodidae) among Forest Fragments and Built Areas in an Endemic Area of American Visceral Leishmaniasis in João Pessoa, Paraíba, Brazil. Insects 2022, 13, 1156. https://doi.org/10.3390/insects13121156
Silva BQd, Afonso MMdS, Freire LJM, Santana ALFd, Pereira-Colavite A, Rangel EF. Ecological Aspects of the Phlebotominae Fauna (Diptera: Psychodidae) among Forest Fragments and Built Areas in an Endemic Area of American Visceral Leishmaniasis in João Pessoa, Paraíba, Brazil. Insects. 2022; 13(12):1156. https://doi.org/10.3390/insects13121156
Chicago/Turabian StyleSilva, Bruna Queiroz da, Margarete Martins dos Santos Afonso, Lucas José Macêdo Freire, Antônio Luís Ferreira de Santana, Alessandre Pereira-Colavite, and Elizabeth Ferreira Rangel. 2022. "Ecological Aspects of the Phlebotominae Fauna (Diptera: Psychodidae) among Forest Fragments and Built Areas in an Endemic Area of American Visceral Leishmaniasis in João Pessoa, Paraíba, Brazil" Insects 13, no. 12: 1156. https://doi.org/10.3390/insects13121156